All published articles of this journal are available on ScienceDirect.
Physicochemical Properties, Antioxidant and Antimicrobial Activities of Sweet Orange (Citrus sinensis L. OSBECK) Fruit Peel and Pulp Oil Extracts
Abstract
Background:
Citrus sinensis L., commonly called sweet orange, fruit waste (peel, seed, and pulp) oils, are used as natural preservatives due to their broad spectrum of biological activities, including antimicrobial and antioxidant effects.
Objective:
The aim of this study was to investigate the physicochemical properties, antioxidant and antimicrobial activities of sweet orange peel and pulp oils extracted using the solvent extraction method.
Methods:
The oil extraction was done in the Soxhlet apparatus using petroleum ether as a solvent. Then, the physicochemical properties of the oil extracts were assessed based on the determination of oil yield, acid value, free fatty acid, and peroxide value. The antioxidant activity of the oil extract was evaluated based on 2,2-diphenyl-1-picrylhydrazyl (DPPH), and hydrogen peroxide free radical scavenging activity as well as ascorbic acid content.
Results:
The results indicated that significantly higher antioxidant activities with respect to ascorbic acid (47.94%) and DPPH value 85.20% were recorded for sweet orange pulp/juice oil. Stronger antibacterial activity with a maximum zone of inhibition (10.67mm), minimum inhibitory concentration (MIC) of 0.25µg/ml, and minimum bactericidal concentration (MBC) of 0.25µg/ml were recorded for fruit pulp oil extract against Staphylococcus aureus. Stronger antifungal activity with a maximum zone of inhibition (9.67mm), MIC (0.25µg/ml), and minimum fungicidal concentration (MFC) of 0.50µg/ml were also observed for fruit pulp oil extract against Aspergillus versicolor.
Conclusion:
C. sinensis fruit pulp oil was found to demonstrate stronger biological activities, including both antioxidant and antimicrobial potentials.
1. INTRODUCTION
Citrus (Citrus spp), belonging to Rutaceae, is a large family whose dominant members include sweet oranges (Citrus sinensis), tangerines/mandarin (Citrus reticulata), lemon (Citrus limon), limes (several species) and grapefruits (Citrus paradis) [1]. Citrus fruit juices are important sources of bioactive materials that are responsible for their medicinal properties, health, and nutritional benefits [2]. The citrus fruit endocarp is rich in soluble sugar, vitamin C, pectin, fibers, organic acids, and potassium salts that are responsible for citrus flavour [3]. Citrus fruits are consumed as fresh or used for making juice, jam, and various types of drinks and food additives. Processing of citrus fruits results in a significant amount of waste (peels, seeds, and pulp) that accounts for 50% of the fruit [4], and is a source of valuable bioactive compounds and essential oils [5-7].
The sweet orange essential oil showed anti-carcinogenic potential via inducing apoptosis in human leukemia (HL-60) cells [8] and human colon cancer cells [8], inhibiting angiogenesis and metastasis [8]. Olfactory stimulation using orange essential oil induced physiological and psychological relaxation. Inhalation of orange essential oil for 90 seconds caused a significant decrease in oxyhemoglobin concentration in the right prefrontal cortex of the brain, which increased comfortable, relaxed, and natural feelings [9, 10]. The odor of sweet orange decreased the symptoms of anxiety and improved the mood [11]. The oil showed strong anxiolytic activity in Wistar rats [12]. When female dental patients were exposed to a sweet orange odor diffused in the waiting room prior to a dental procedure, they showed lower levels of state anxiety compared to control patients who were exposed to air only [13].
The sweet orange essential oil in combination with ginger and accompanied by a massage was effective in alleviating moderate to severe knee pain among the elderly in Hong Kong [14]. Moreover, sweet orange essential oil suppressed pre-neoplastic hepatic lesions during N-nitrosodiethylamine (DEN)-induced hepatocarcinogenesis in rats by restoring the normal phenotype and upregulating junctional complexes [15]. Injections of orange essential oil in mice 24 h after subcutaneous injections with dibenzo-[α]-pyrene (DBP) reduced the tumor incidence to less than 50% after 30 weeks [16]. In addition, the oil was reported to have a good radical-scavenging activity [17], mainly due to the high d-limonene content [18, 19].
The availability widespread availability of citrus fruit wastes provides large opportunities for their application in sustainable pharmaceutical, nutraceutical, food, and beverage industries. The peel of citrus fruit has numerous glands that contain oil that is typically recovered as a major by-product [20]. Citrus fruit has its characteristic set of compounds that comprise the oil and that are responsible for its flavor and aroma. Various extraction methods, including supercritical fluid extraction, microwave-assisted extraction, and Soxhlet method, have been used for oil extraction. The use of appropriate extraction technology, plant materials, manufacturing equipment, and solvent type is important to obtain quality oil. In Ethiopia, limited studies exist on citrus fruit production, management and market values despite large consumption and demand. Therefore, the aim of this study was to investigate the physicochemical properties, and antioxidant and antimicrobial activities of sweet orange peel and pulp oils extracted using the solvent extraction method.
2. MATERIALS AND METHODS
2.1. Sample Collection, Preparation, and Oil Extraction
The experiment was conducted in the Molecular biology and Biotechnology Lab, Haramaya University. The sweet orange (Citrus sinensis L.) fruit sample was collected from Toni Farm in Dire Dewa City, Ethiopia. The fruit peels and pulp samples were freeze-dried and grinded in a grinder. Then, the oil extraction was conducted in a soxhlet apparatus using petroleum ether as a solvent.
2.2. Physicochemical Properties of the Oil Extracts
The oil yield and specific gravity were determined based on the solvent extraction method [21]. The percentage oil yield of each sample was determined as follows:
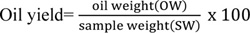 |
Where, oil weight= W2-W1; W1= weight of the extraction flask (g), W2= weight of the extraction flask plus the dried crude fat (g).
The specific gravity of the oil was determined gravimetrically by employing the weight ratio of the oil to the equivalent amount of water according to the following formula:
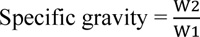 |
Where, W2 and W1 are the weights of oil and an equivalent amount of water, respectively.
2.2.1. Determination of Acid Value
The acid value was determined as per AOAC [22] method. Briefly 2g of oil sample was weighed into a 250ml conical flask, and then, 25ml diethyl ether mixed with 25ml alcohol and 1ml of 1% phenolphthalein indicator was added to the oil sample. The conical flask was then placed in a hot water bath until the oil was completely dissolved in the solvent. The hot solution was then titrated with 0.1M KOH until a pink colour that persisted for 15 seconds was noticed. The acid value was calculated as:
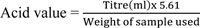 |
Acid value is expressed as mg KOH/g of oil.
2.2.2. Estimation of Free Fatty Acid
The percentage of free fatty acid (%FFA) was estimated by multiplying the acid value by the factor 0.503. The %FFA = 0.503× acid value.
2.2.3. Determination of Peroxide Value
To a weighed sample (1.0g) in a flask was added powdered potassium iodide (1.0g) and solvent mixture (2: 1, glacial acetic acid:chloroform v/v). The resulting solution was then placed in a water bath to dissolve properly, and 5% potassium iodide (20cm3) was then added. The sample solution was then titrated with 0.002N sodium thiosulphate using starch as an indicator. The peroxide values of the samples were calculated using the equation below [23].
PV = 2 X V
Where, PV = peroxide value, V = volume of sodium thiosulphate used, 2 = (N x 1000) / W, N = normality of sodium thiosulphate used, and W = weight of sample used.
2.3. Antioxidant Activity
2.3.1. DPPH Radical Scavenging Activity
The radical scavenging activity (RSA) of the oil extract is used to measure antioxidant activity using the DPPH (2, 2- diphenyl-1-picrylhydrazyl) method [24]. Briefly, 2 mL of oil extract (100µg/ml) was added to 2mL of DPPH (0.1 mM) solution. The mixture was kept aside in a dark area for 30 min, and absorbance was measured at λmax 517 nm against an equivalent amount of DPPH with methanol used as a blank. The percentage of DPPH radical scavenging activity (RSA %) was estimated using the equation:
DPPH radical scavenging activity(%)=

Where, Ablank is the absorbance of the blank solution, and Asample is the absorbance in the presence of the sample. Ascorbic acid was used as the positive control.
2.3.2. Hydrogen Peroxide Scavenging Activity
The radical scavenging activity of the oil extract was determined using the H2O2 method [25]. Briefly, 2ml of oil extract solution (100µg/ml) was added to 4.0ml of H2O2 (20 mM) solution in phosphate buffer (pH 7.4). After 10 min, the absorbance was measured at λmax 230 nm against the phosphate buffer blank solution. The percentage scavenging of H2O2 was calculated using the equation:
% scavenging of H2O2

Where, Ablank = absorbance of the blank (phosphate buffer with H2O2) and Asample = absorbance of the oil extracts.
2.3.3. Ascorbic Acid Content
The ascorbic acid content was determined using the 2, 6- dichlorophenol indophenol (DCPIP) dye method [22]. Accordingly, 5ml of the standard ascorbic acid solution was pipetted into a 100 ml conical flask, and then 5ml of the 3% HPO3 solution was added. The ascorbic acid solution was titrated with the dye solution to a pink colour that persisted for 15sec. The titer value was recorded. Dye factor was expressed as mg of ascorbic acid per ml of the dye, since 5ml of the standard ascorbic acid solution contained 0.5 mg of ascorbic acid:
Dye factor (mg ascorbic acid per dye)
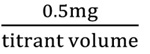
1 ml of the extracted oil was diluted to 5ml with 3% metaphosphoric acid in a 50 ml volumetric flask. The aliquot was then centrifuged (Model, Z300, 580W, 3052 Nm, Germany) for 15 minutes and titrated with the standard dye to a pink endpoint (persisting for 15 seconds). The ascorbic acid content was calculated from the titration value, dye factor, dilution and volume of the sample as follows:
% A.A

Where, A.A = ascorbic acid, ABR= average burette reading.
2.4. Antimicrobial Activity
The antimicrobial experiment was carried out as a three-factorial experiment, including 2 source extracts: sweet orange fruit peel and pulp oils at three concentration levels; petroleum ether as a solvent; and four test pathogens, including two bacteria, Escherichia coli (gram-negative) and Staphylococcus aureus (gram-positive), and two fungi (Aspergillus versicolor and A. Niger), completely randomized in three replications. The test pathogens were obtained from the Ethiopian Institute of Food and Public Health.
The turbidity of each bacterial and fungal spore suspension matched the turbidity of 0.5 McFarland standards using saline solution [26]. Discs of 6 mm diameter were prepared from sterile filter paper cut into small circular pieces of equal size by a perforator, and then each of them was impregnated with 0.01 ml of the prepared test extract ethyl acetate solution. The extract impregnated discs were placed onto Muller Hinton agar (MHA) plates evenly inoculated with test pathogens. Ketoconazole (1µg/disc) disc was applied as a positive control and distilled water served as a negative control for incubation of fungi, while amoxicillin (1µg/disc) served as a positive control and distilled water as a negative control for bacterial pathogens.
The oil extracts that showed significant antimicrobial activity in the antimicrobial activity tests were selected for determination of MIC based on the broth dilution method used by Mousavi et al. [27] with slight modifications. Briefly, 0. 2ml of the prepared concentration of each oil extract was mixed with 2ml of nutrient broth and potato dextrose agar medium. Thereafter, standardized inoculums of 0.1 ml of the respective test pathogens were dispensed into the test tubes containing the suspensions of the broth and the oil extract. Then, all test tubes were properly corked and incubated at 37°C for 24 hrs for bacteria and 27°C for 72 hrs for fungi. After that, they were observed for the absence or presence of visible growth.
The minimum bactericidal concentration (MBC) or minimum fungicidal concentration (MFC) values were determined by subculturing from respective MIC values. The MBC plates were incubated for 48 h, and MFC assay plates were incubated for 3 days. After the incubation periods, the lowest concentration of the extract that did not allow any bacterial or fungal growth on a solid medium was regarded as MBC and MFC for the extract.
2.5. Data Analysis
The experimental data were analyzed using SAS version 9.2 [28]. to investigate statistical significance between the different oil quality parameters. Differences between means were considered statistically significant at P< 0.05.
| Oil extract | Oil yield (%) | ACV(g/L) | FFA(%) | PV(meq/kg) |
|---|---|---|---|---|
| Pulp | 48.75±3.54b | 0.98±0.20b | 0.49±0.10b | 0.17±0.03a |
| Peel | 67.50±1.77a | 2.38±0.60a | 1.20±0.30a | 0.18±0.03a |
3. RESULTS AND DISCUSSION
3.1. Physicochemical Properties of Sweet Orange (Citrus sinensis L.) Oil Extracts
The physicochemical properties of Citrus sinensis oil extracted from ripened peel and pulp are presented in (Table 1). Significantly higher oil yield (67.50%), acid value (2.38g/L), and free fatty acid value (1.20%) were recorded for peel oil extract. However, no significant difference in peroxide value between pulp and peel oils was observed.
3.2. Antioxidant Activities of Sweet Orange Fruit Peel and Pulp Oil Extracts
Antioxidant activities with respect to ascorbic acid content, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and hydrogen peroxide scavenging activity were used to screen the radical scavenging activity of oil extracted from C. sinensis peel and pulp oil (Table 2). It was observed that significantly higher ascorbic acid (47.94±0.94%) and DPPH (85.20±2.83%) were recorded for fruit pulp/juice oil. However, significantly higher hydrogen peroxide scavenging activity (11.50±1.41) was recorded for sweet orange peel oil, indicating that sweet orange juice exhibited more quality than peel oil. This finding was in agreement with Barreca et al. [13], who reported that the total antioxidant activity of C. sinensis crude juice scavenged DPPH radicals with an antioxidant power corresponding to 14.39±0.19 µM; Trolox equivalents (TE) were used as the reference.
| Oil extract | DPPH | HPSA | AA (%v/v) |
|---|---|---|---|
| Fruit pulp | 85.20±2.83a | 6.00±2.12b | 47.94±0.94a |
| Fruit peel | 14.00±1.70b | 11.50±1.41a | 44.57±1.01b |
3.3. Antimicrobial Activity of Sweet Orange (Citrus sinensis L.) Fruit Peel and Pulp Oils
The oil extract from C. sinensis fruit peel and pulp indicated considerable antibacterial activity based on disk diffusion assay (Table 3). Susceptibility of the two tested bacteria and fungi to the oil extracts has demonstrated a significance difference between C. sinensis pulp and peel oil extracts. Oil extracts with colony growth inhibitory effect at the highest dose showed a mean zone of inhibition ranging from 9.00 to 10.67mm. Amoxicillin (used as a positive control) showed a significant superiority (p<0.05) in the zone of inhibition compared to the test extracts (Table 3). For most of the test extracts, the highest concentration (2.0µg/mL) exhibited a significantly higher (P<0.05) zone of inhibition compared to the respective lowest concentration (1.0µg/mL). The strongest antibacterial activity with a maximum zone of inhibition (10.67mm) was recorded with pulp oil extract against S. aureus at 2.0µg/ml of concentration, indicating that S. aureus is more susceptible to the oil extracts than E. coli.
The sweet orange essential oil was reported to inhibit the growth of both gram-negative and gram-positive bacterial spp [30-32, 34]. as well as several fungal spp [34-36]. It also showed good anti-aflatoxigenic effects (inhibited aflatoxin B1) at 500 ppm [36]. In addition, it exerted an intense larvicidal activity against the malaria vector, Anopheles labranchiae [37], and the vector of yellow and dengue fever, Aedes aegypti [38]. Sweet orange essential oil is a potent fumigant against house flies, cockroaches, and mosquitoes [39, 40]. It can be used for controlling subterranean termites [41]. It is also an effective anthelmintic agent against gastrointestinal nematodes, five times more effective on Haemonchus contortus eggs than tea tree essential oil [42]. Moreover, sweet orange essential oil acted as a growth promoter, increased immunity, and improved disease resistance to Streptococcus iniae in Tilapia [43].
|
Pathogen (Bacteria/Fungi) |
Oil extract | Concentration of oil extract |
Amoxicillin (1.0µg/ml) |
||
|---|---|---|---|---|---|
| 1.0µg/ml | 1.5µg/ml | 2.0µg/ml | |||
| Escherichia coli | Pulp | 7.67±0.58aD | 9.17±0.76aC | 10.50±0.50abB | 18.50±0.49aA |
| E. coli | Peel | 0.00±0.0bD | 7.17±0.76bC | 9.00±1.0bB | 19.17±0.29aA |
| Staphylococcus aureus | Pulp | 7.17±0.29aD | 8.83±0.27aC | 11.00±1.15aB | 19.00±0.50aA |
| S. aureus | Peel | 0.00±0.0bD | 6.50±0.87bC | 10.00±0.5abB | 18.50±0.50aA |
| Ketoconazole (1.0µg/ml) |
|||||
| Aspergillus niger | Pulp | 7.50±0.50aD | 8.00±0.50aC | 9.50±0.76aB | 18.67±0.58aA |
| A. niger | Peel | 0.00±0bD | 7.00±0.51aC | 8.50±0.50bB | 18.83±0.29aA |
| A. versicolor | Pulp | 8.00±0.0aD | 8.50±0.5aC | 10.50±0.52abB | 18.67±0.29aA |
| A. versicolor | Peel | 0.00±0aD | 7.83±0.29aC | 9.50±0.50bB | 18.33±0.76aA |
The antifungal activity of the oil extracts demonstrated that the colony growth inhibitory effect at the highest dose showed a mean zone of inhibition ranging from 8.50 to 9.67 mm. Ketoconazole (used as a positive control) showed a significant superiority (p<0.05) in the zone of inhibition compared to the test oil extracts (Table 3). For most of the test extracts, the highest concentration (2.0µg/mL) exhibited a significantly higher (P<0.05) zone of inhibition compared to the other lower concentrations (1.0 and 1.50µg/mL). The strongest antifungal activity with a maximum zone of inhibition (9.67mm) was recorded with pulp oil extract against A. niger at 2.0µg/ml of concentration, indicating that A. niger is more susceptible to the oil extracts than A. versicolor. A similar study was conducted by Singh et al. [44], who reported the maximum antibacterial activity of fruit peel extract for Staphylococcus aureus (15.9 mm), followed by Escherichia coli (14.0 mm), and maximum antifungal activity for Candida albicans (12.9 mm), followed by Aspergillus niger (12.3 mm).
3.4. Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and Minimum Fungicidal Concentration (MFC) of the Oil Extracts
The MIC, MBC, and MFC tests for sweet orange peel and pulp are shown in Table 4. The strongest antibacterial activity was recorded for pulp oil with the least MIC (0.25µg/ml) and MBC (0.25µg/ml) against S. aureus, indicating S. aureus as more susceptible to the oil extract, while the weakest antibacterial activity (with the largest value of MIC) was observed for peel oil with MIC (0.50µg/ml) and MBC (1.0µg/ml) against E. coli, showing E. coli as more resistant to the oil extract. It can also be observed from this finding that pulp oil was a more effective antimicrobial than peel oil in C. sinensis fruit.
Likewise, the oil extracted from sweet orange pulp exhibited strong antifungal activity with the least MIC (0.25µg/ml) and corresponding MFC (0.50µg/ml) against A. versicolor, showing that A.versicolor was more susceptible to the oil extract. The weaker antifungal activity with the highest MIC (1.0µg/ml) and MFC (2.0µg/ml) was recorded for peel oil against A. niger that indicated A. niger as less susceptible to the oil extract.
| Microbes (Bacteria/Fungi) | Oil extract | MIC (µg/ml) | MBC/MFC (µg/ml) |
|---|---|---|---|
| E. coli | Peel | 0.50 | 1.00 |
| Pulp | 0.25 | 0.50 | |
| S. aureus | Peel | 0.25 | 0.75 |
| Pulp | 0.25 | 0.25 | |
| A. niger | Peel | 1.0 | 2.0 |
| Pulp | 0.50 | 1.0 | |
| A. versicolor | Peel | 0.50 | 0.50 |
| Pulp | 0.25 | 0.50 |
CONCLUSION
The results with respect to physicochemical properties indicated higher acid value, free fatty acid, DPPH, and ascorbic acid content of pulp/juice oil extract, indicating that sweet orange juice exhibited more quality than peel oil. The antimicrobial activities of oil extracts demonstrated higher antibacterial and antifungal activities for pulp oil. It can be concluded from the present study that higher oil quality with antioxidant (ascorbic acid) and DPPH radical scavenging activity exhibited better antimicrobial activity. Thus, higher oil quality is directly correlated with strong antimicrobial activity. Such a concept generates good information for medicinal plant breeders and horticulturalists.
AUTHORS’ CONTRIBUTIONS
Zekeria Yusuf contributed to initiation and design of the study, lab experiment, and data analysis; Muhammednur Sado and Megersa Idris performed lab experiment, data collection, and wrote the document; Mulugeta Desta contributed to snalysis and interpretation of data. All authors contributed to drafting of the article and revising it critically for important intellectual content.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animal/humans were used that are the basis of this study.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data material will be made available on request from corresponding author [Z.Y].
FUNDING
This project was funded by Haramaya University Research grant, under project code: HUIF_2019_06_01_98.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to Haramaya University Research Office for their financial support and laboratory facilities.


