All published articles of this journal are available on ScienceDirect.
Morphological and Proteomic Evaluation of Zea Mays in Response to Osmotic Stress
Abstract
Introduction:
Drought is the main abiotic stress responsible for crop loss worldwide. Maize (Zea mays L.) is a widely grown drought-sensitive crop used as a staple food by the growing population. Therefore, it is imperative to assess the molecular mechanisms behind drought response and tolerance in maize. Transcriptomic profiling of abiotic stress responsive pathways in various crops appeared to be an unreliable approach due to post-transcriptional modifications, while there is limited published data on molecular mechanisms of osmotic-stress response in maize. Hence our study aimed at profiling osmotic stress responsive proteins augmented by their associated morphological features in Z. mays.
Materials and Methods:
In this regard, morphological and proteomic investigations were carried out on 16-day maize seedlings exposed to 5% (w/v) and 10% (w/v) polyethylene glycol(PEG) to induce osmotic-stress. Proteomics approach (one-dimensional (1D) and two-dimensional (2D) gel electrophoresis) compared differential protein abundance between controls and the osmotic stressed maize plants.
Results:
Morphological parameters such as plant growth, height, shoot diameter, leaf area, and colour were highly affected with PEG treatment as compared to the untreated ones. Molecular evaluation by 1D gel electrophoresis revealed that the separated protein patterns were highly expressed in the experiments than the controls. Using 2D gel electrophoresis, a total of seven and eight protein spots were revealed in experimental plants under 5% (w/v) and 10% (w/v) PEG treatment respectively while the control plants only expressed one protein. Increased drought stress resulted in a greater number of proteins with differential abundance.
Conclusion:
This study has successfully profiled the total osmotic stress responsive proteins and revealed the efficiency of proteomic tools in the qualitative detection of differential proteins from maize.
1. INTRODUCTION
Plants are often exposed to various biotic and abiotic stress factors. Abiotic stresses, such as drought, extreme heat, freezing, heavy metals, and high salinity, result in diminished plant growth and loss of crop productivity world-wide [1].The majority of these environmental conditions impose osmotic-stress that has been demonstrated by reduced water potential and severe impact on plant development [2]. Osmotic-stress has detrimental effects on plant development that manifest in the inhibition of cell elongation, stomatal closure, reduction of photosynthetic activity, disturbances in water and ion uptake as well as changes in metabolic processes [3, 4].
Drought, salinity and low temperature are main stress factors commonly known to affect agricultural crops. Conceivably, this study focused on drought as an abiotic factor that induces osmotic-stress. Notably, drought is one of the major stress factors that affect agricultural crops more than any other stress and is becoming even more severe in many parts of the world [5]. It does not only cause differences between the mean yield and potential yield, but also the overall year to year yield. Plants exposure to drought results in excessive formation of reactive oxygen species (ROS) that cause cell death and reduced growth.
Globally, 52% of the population relies on cereals for human nutrition. Maize (Zea mays L.) is one of the main cereals grown world-wide and can be cultivated under a variety of environment conditions [6]. Besides, maize is a significant staple food for about over 1.2 billion people in the world, providing for more than 20% of the total calories in 21 countries’ human diets. This cereal also serves as a commercial crop, which is highly sensitive to drought [7]. Being a drought-sensitive crop, maize is affected at each stage of its growth and development by lesser moisture availability. Various physiological traits such as reduced photosynthetic rates and shutting down of plant metabolism are normally succeeded by plant death due to stomatal closure and inhibited gaseous exchange which, occur in response to prolonged or moderate drought stress [8]. It has been predicted that by the year 2050, the global demand of maize will double in the developing world due to its potential as a crop with the greatest production [9]. Hence the global importance of maize and the associated effects of drought triggered plant breeders to develop drought-tolerant maize germplasm. Drought responsive traits and adaptive mechanisms must be known for the development of drought-tolerant maize stocks. To date, various studies have reported the adverse effect of water shortage on germination and seedling growth in different crops [10, 11].
In most laboratories, solutions of high molecular weight such as polyethylene glycol (PEG), has been used to control water potential in seed germination studies [12] and the PEG inhibition of germination has been attributed to osmotic stress [13, 14]. In order to look into drought stress induced proteins, different concentrations of PEG 8000 were used as osmoticum to investigate the status of leaf morphology and proline pools in maize seedlings [12]. The increase in the accumulation of salts and ions in the upper layers of the soil around the root causes osmotic-stress and ion toxicity [13]. Osmotic-stress is mostly aggravated by drought, which remains one of the major abiotic factors that limit worldwide productivity and distribution of cereal crops such as maize. Osmotic-stress induced proteins in plants have a negative impact on the ecosystem and agriculture and this normally results in loss of crop yield worldwide that ultimately would lead to food insecurity. Several studies have been conducted in maize that includes mechanisms of crop development and environmental adaptation to drought, in order to improve quality and yield [14]. To understand the molecular mechanisms by which plants use to respond to osmotic stress, transcriptomic studies have been conducted in root tissues of various plant species. However, transcriptomic studies appeared to be an unreliable method due to post-transcriptional and post-translational modifications [15-17]. Zorb and co-workers (2010) studied proteomic changes in maize roots after a short-term adjustment to saline growth conditions and found such studies so reliable [18]. Currently there are a limited number of studies performed on proteomic profiling of maize and hence this study was therefore, designed to investigate the effect of osmotic stress (as a result of PEG treatments) on leaf morphology, and the expression profiles of stress responsive proteins in a Z. mays cultivar,R450 w/uo2550 CML550. Information to be obtained from this study was set to create a platform from which further studies on the identification of osmotic induced proteins in maize could be undertaken, leading to a better understanding of their role in plant stress response and adaptation mechanisms. Furthermore, the study was also set to assist approaches in plant biology and providing solutions to the management of osmotic-stressed crop plants.
2. MATERIALS AND METHODS
2.1. Plant Material and Osmotic Stress Treatment
Changes in morphology and protein expression patterns in response to drought stress were studied, using an R450w/uo2550w CML 550 (drought-sensitive) Zea mays seed cultivator obtained from Molelwane farm, Department of Crop Science, North-West-University, RSA. About four seeds per plant pot were placed in a 50 ml falcon tube, where 2 ml of 70% (v/v) ethanol was used for surface sterilization of the seeds for 1 minute, followed by further sterilization with 1.25% (v/v) commercial bleach for 10 minutes. Immediately after surface decontamination, seeds were rinsed thrice with sterile distilled water to remove traces of ethanol and bleach. The sterilized seeds (4 per plant pot), were sown in eighteen, 20 cm plastic plant pots with sterile potting soil composed of 3 parts peat-based soil and 2 parts vermiculite, and watered with sterile tap water daily. The seeds were then allowed to germinate and grow under long days (16-hour days) and short nights (8-hour nights) at a constant temperature of 25°C and relative humidity of 75%. All seedlings were watered with 100 ml sterile tap water. Immediately after 8 days of germination, maize plants were divided into three independent experimental batches (3 plant pots per treatment) in a randomized design to eliminate any variation due to environmental conditions. The control plants (untreated) were supplied with sterile tap water at intervals of 2 days for a total of 16 days. Experimental plants (2 treatment groups) were supplied with either 5% (w/v) or 10% (w/v) PEG solutions at an interval of 2 days for 16 days under the same growth conditions. Both the control and experimental batches were performed in triplicate and allowed to grow under the same growth conditions. Germination rates for all the plant groups were recorded daily up until the treatment was stopped. After 16 days of treatment, plant leaves from both the control and experimental seedlings were harvested and rinsed with sterile distilled water, followed by snap freezing in liquid N2 and storage at -80°C until further processing.
2.2. Total Protein Extraction and Purification for Electrophoresis
Total protein extraction from the leaves of the 16-day old maize seedlings of both the control and experiment plants were performed following the method described by [19]. The frozen maize leaf material from all the three independent treatments were utilized, where about 1 g of leaf material was briefly ground into fine powder using liquid nitrogen and transferred into sterile Eppendorf tubes. The powdered leaf material was suspended in 500 μl of 10% (w/v) trichloroacetic acid (TCA) for precipitation. The homogenates were centrifuged at 13,400 x g, 4°C for 10 minutes using a Tomos High speed microcentrifuge (model: MultiStar 21, Hermle Labortechnik, Wehingen, Germany). After centrifugation, supernatants were discarded and the remaining pellets were washed three times with 1 ml of 80% (v/v) ice cold acetone through centrifugation at 13,400 x g for 10 minutes per wash. The pellets were air dried for 5 minutes at room temperature, followed by resuspension in 800 μl of urea lysis buffer (9 M urea, 2 M thiourea and 4% (w/v) 3-cholamidopropyl dimethylammonio 1-propanesulfonate (CHAPS)) for an hour with vigorous vortexing at room temperature. After a vigorous one hour vortexing, the homogenates were centrifuged at 15,700 x g for 10 minutes, followed by collection of the supernatants containing the soluble leaf protein fractions into sterile Eppendorf tubes. Furthermore, the obtained total leaf protein fractions were purified using a ReadyPrep™2-D Cleanup kit (catalog # 163-2130, Bio-Rad Laboratories Inc., California, USA) and according to the manufacturer’s instructions. The protein concentrations were measured using a 2000 Nanodrop spectrophotometer (Thermo Scientific Inc., California, USA) and expressed as μg/ul.
2.3. One-dimensional Electrophoresis (1-DE) of Total Soluble Proteins
One-dimensional electrophoresis (1-DE) was performed following procedures described by [19]. Briefly, a one-dimensional (1D) sodium dodecyl-polyacrylamide gel electrophoresis (SDS-PAGE) was prepared from a 12% (v/v) running gel and a 4% (v/v) stacking gel. About 5 µl of unstained protein marker (Catalog# P7704S New England Biolabs Inc., Massachusetts, USA) was run alongside 10 µg of the extracted total leaf protein samples and electrophoresed at 200 volts until the dye front had reached the end of the bottom gel. Proteins were then stained with Coomassie brilliant blue R-250, followed by destaining for 45 minutes.
2.4. Two-dimensional Electrophoresis (2DE) of Total Soluble Proteins
All positively induced proteins, profiled on the 1DE between controls and treatments were identified and selected for further applications in 2 dimensional gel electrophoresis (2DE). In the first dimensional isoelectric focusing gel, total soluble protein samples of about 200 µg were resuspended in the rehydration buffer (2% (w/v) CHAPS, 50 mM (w/v) dithiothreitol (DTT), 0.2% (v/v) ampholytes, 0.1% (w/v) bromophenol blue (Bio-Rad Laboratories Inc., California, USA). The samples were passively rehydrated on linear 7-cm immobilized pH 3-10 gradient gels (Bio-Rad Laboratories Inc., California, USA) overnight at room temperature. On a subsequent day, the rehydrated strips were transferred to a focusing tray and subjected to isoelectric focusing (IEF) on a PROTEAN i12 IEF cell (Bio-Rad Laboratories, California, USA) in a step wise program as described by [19]. After isoelectric focusing, the strips were equilibrated twice in an equilibrium buffer (6 M urea, 2% (w/v) SDS, 0.35 M Tris-HCL, pH 8.8 and 20% (v/v) glycerol); firstly, containing 2% (w/v) DTT followed by 2.5% (w/v) iodoacetamide, with gentle agitation on an orbital shaker (catalog# 12020970, Labnet International Inc. Tokyo, Japan) for 10 minutes per equilibration. The equilibrated strips were then immersed in a 100 ml graduated cylinder with 1x Tris-glycerine-SDS (TGS) running buffer, followed by their insertion onto 15% (w/v) polyacrylamide resolving gels. The inserted strips were overlaid with melted agarose solution (100 ml 1 x SDS-PAGE running buffer; 0.5% (w/v) agarose; 0.002% (w/v) bromophenol blue) and allowed to polymerize. The sealed gels were then electrophoresed on a mini-PROTEAN Tetra system (Bio-Rad Laboratories Inc., California, USA) at 180 volts until the dye front had reached the bottom part of the gel plates as described by [20]. After electrophoresis, the gels were stained in a Coomassie brilliant blue R-250 solution for 50 minutes, followed by destaining (100% ethanol, 100% methanol, 100% acetic acid), shaking on an ultra-rocker (Bio-Rad Laboratories., USA) until the protein spots were visible. The gels were imaged with a Chemi DOCTM Imaging system (Bio-Rad Laboratories Inc., California, USA) using the Bio-Image LabTMsoftware.
3. RESULTS
3.1. Morphological Effect of Drought-Stress on Zea Mays
Changes of morphological and developmental patterns of Z. mays R450 w/uo 2250w CML550 cultivar were compared between the three sets of plants exposed to osmotic-stress: control plants (water only), 5% (w/v) PEG treated plants and 10% (w/v) PEG treated plants. Osmotic-stress has shown noticeable changes in the general morphology of the developed Z. mays seedlings. The phenotypic differences between the three sets of plants were recorded for 16 days as shown in (Fig. 1). Several drought-induced morphological changes were observed, such included delayed plant development in experimental plants as compared to the control plants. The leaves of experiments 1 and 2 were distinctly pale green and wrinkled with narrow leaf blades (Fig. 1B and C), whilst the control seedlings displayed no phenotypic change by maintaining fully expanded green leaves and intact plant structures (Fig. 1A and D). The stem diameter of controls (Fig. 1A and B) was thicker as compared to the experiments (Fig. 1B-F). The plant height of the control seedlings (Fig. 1D) indicated an elongation as compared to the experiments (Fig. 1E and F) with diminished heights.
3.2. Separation of Osmotically Stressed Maize Proteome
To evaluate the quality of maize proteome under osmotic-stress, separation of the total soluble protein extracts was undertaken. Purified protein leaf extracts were separated by 1DE followed by comparison of the stress induced proteins with the non-induced ones as is shown in (Fig. 2). Protein expression patterns showed the presence/absence of high and lower protein bands for both the control and osmotic stressed treatments. The protein extracts exhibited a mixture of varying protein expression patterns, abundance and loading across replicates for both the control and osmotic-stressed treatments (Fig. 2). Furthermore, a difference in protein profile of the osmotic-stress proteins were observed in E1 (5% (w/v) PEG) and E2 (10% (w/v) PEG) (25, 30, 40, 58, 180 and 250 kDa) as compared to the control (C1 and C2), in which such proteins were absent (Fig. 2). The expression profiles for both treatments remained the same despite the varying concentrations of PEG.

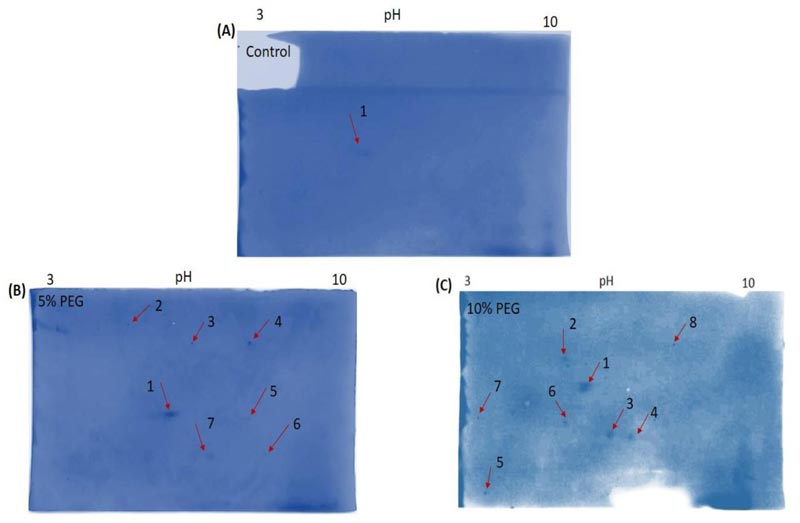
3.3. Osmotic Stress Responsive Protein Profiles from Z. Mays using Two-dimensional (2D) Gel Electrophoresis Analysis
Following the resolution of the expressed protein profiles in the 1D SDS-PAGE, only good quality purified proteins were carefully selected and used for 2D SDS-PAGE analysis for the detection of osmotic-stress responsive proteins in maize leaf extracts. Purified protein extracts from the 16-day old PEG treated seedlings were resolved on 7 cm IPG strips, pH range 3-10. The loaded protein extracts were separated on a 12% (w/v) SDS-PAGE as indicated in (Fig. 3), and protein abundance among the three biological replicate gels (not shown) for each sample was uniform. The protein profile in the control (water only) indicated a single protein spot as compared to the experimental groups (5% (w/v) and 10% (w/v) PEG treatments) that demonstrated numerous protein spots with PEG induction illustrating the influence of osmotic-stress on the expression of various proteins (Fig. 3). An overall of eight differentially expressed protein spots were observed between the PEG treated and water only treated leaf extracts (Fig. 3).

4. DISCUSSION
Osmotic-stress occurs as a result of several abiotic stress factors such as drought, high salinity, and low temperature that cause severe cell dehydration and adversely affecting plant growth and development [21]. As one of the most serious environmental stresses for plants, drought adversely affects plant growth and development, limiting crop production more than any other environmental factor [22]. Compatible osmolytes are powerful cytoprotectants that plays a major role against the effects of osmotic-stress induced by drought in plants [23]. During abiotic stress, drought effects on maize may change their gene expression of protein accumulation. It is widely known that there are multiple transient responses to environmental shock and that so many genes are common to several types of stresses such as cold, salinity, heat and drought [23]. Plant adaptation to environmental stresses is controlled by a number of molecular networks, resulting in a combination of metabolic, physiological and morphological changes [24-26]. In general, the response to drought stress varies with the plant species, genotype, developmental stage and severity of stress. Therefore, to develop plants with enhanced tolerance against drought, a thorough knowledge of the physiological, biochemical and molecular networks is essential. Polyethylene glycol (PEG) has been widely used in various studies to investigate the osmoadaptive responses during growth at different osmotic pressures [24].
To understand the molecular changes that occur in maize (Zea mays L.) crops during drought-stress, morphological and proteomic approaches were used to screen for proteins involved in drought-stress response. PEG has always been used as the treatment of chemical to induce drought-stress in plants in a controlled manner. In our study, 5% (w/v) and 10% (w/v) of PEG solutions were used as treatment variables to induce drought-stress against the control (water only) in Z. mays. Our work aimed at investigating how osmotic stress (as consequence of PEG osmotic stress induced) treatments influence the morphological and proteomic parameters of maize plants. It further presented information on the profiles of stress responsive proteins associated with hyperosmotic stress using the 1 DE and 2-DE techniques. The information gathered here provided an understanding of the abiotic (osmotic) stress responses in plants and give insight into possible approaches to develop well adapted maize or related crop plants against osmotic pressures.
The influence of osmotic-stress on Z. mays was actually determined by exposing 8 days old seedlings to various treatments, where the control was irrigated with water only, then experiments with 5% (w/v) PEG (experiment 1) and 10% (w/v) PEG (experiment 2) for 16 days. Several drought-induced morphological changes were observed. A decrease in the number of leaves in experimental seedlings was noticed in comparison to the increased number in control plants (Fig. 1). The leaves of experiments 1 and 2 were distinctly wrinkled with narrow leaf blades (Fig. 1B and C), whilst the control seedlings displayed little/no phenotypic change; by maintaining fully expanded green leaves and intact plant structures (Fig. 1A and D) . Also, the plant height of the control seedlings (Fig. 1A and D) showed an elongation as compared to the experiments (Fig. 1E and F) with diminished heights. Experimental seedlings exhibited thin stems (Fig. 1A and D) as compared to the control seedlings with strong thickened stem diameters (Fig. 1B and C). In general, there were no evident phenotypic changes between the varying concentrations of the PEG treated seedlings (5% (w/v) and 10% (w/v)), illustrating the effect of osmotic stress on developing plants. Our results support a previous report, whereby hyperosmotic stress was found to have a major influence in plant growth parameters such as a reduction of the shoot and root length [5].
To further conceptualize the effect of osmotic-stress on Z. mays, proteomic analysis was carried out. One dimensional (1D) sodium dodecyl-polyacrylamide gel electrophoresis (SDS-PAGE) was used as the first step to analyze the extracted total leaf proteins expressed under simulated osmotic-stress (5% (w/v) and 10% (w/v) PEG) compared to the control (water only). Separation of proteins by 1DE is essential for resolving total proteome according to their molecular weight [27]. In this regard, one dimensional gel electrophoresis (1DE) of the protein extracts demonstrated that protein expression and abundance was uniform in both the control (un-stressed) and experimental plants (stressed) for all the biological replicates (Fig. 2). Proteins from the control seedlings were partially expressed as compared to the treated samples, which abundantly expressed proteins ranging from 25 to 250 kDa (Fig. 2). The control proteins were partially/not expressed as compared to the treated samples (experiment 1 and 2), where protein bands were more pronounced in the 250, 58, 30 and 25 kDa range (Fig. 2) due to stress induction. Our findings concur with the previous investigations, where an accumulation of total soluble proteins were observed with an increased PEG concentration in barley genotypes [28]. Changes in protein abundance, accumulation and synthesis have been observed in rice, after exposure to osmotic-stress with PEG [29].
The purified total soluble proteins were analysed by 2D gel electrophoresis (2DE). This 2DE is normally used to resolve proteins according to their isoelectric points (pI) and molecular weights [30, 31]. In our case and to significantly assess the expression profiles of leaf protein extracts between the stressed (5% (w/v) and 10% (w/v) PEG) and unstressed (water) 16-day old maize plants, a comparative 2DE proteomic analysis was thus conducted, whereby 200 μg protein extracts from three independent replicates between the stressed and unstressed leaves were resolved on 7cm IPG strips of pH range 3-10.The control (water only treatment) showed the separation of only one Coomassie stained protein spot (Fig. 3A) whereas in experiment 1 (5% (w/v) PEG treatment), seven protein spots were visible and in experiment 2 (10% (w/v) PEG treatment), approximately eight spots were visible (Fig. 3B and C). Results obtained indicate that increased concentrations of PEG induce osmotic stress and result in the expression of most proteins as compared to the control. A total of eight protein spots were differentially expressed in response to PEG osmotic-stress as compared to the control, where only one protein spot was expressed (Fig. 3). In (Fig. 3B), seven protein spots were differentially induced by 5% (w/v) PEG, while there was an up-regulation of protein spot 1 as compared to the control. At 10% (w/v) PEG treatment, eight protein spots were induced with an increased up-regulation of the same protein spot 1, which shows that with an increased osmotic-stress, more proteins were expressed (Fig. 3C).
Overall, our results indicate that PEG osmotic-stress induced abundant proteins from Z. mays leaves as compared to the control (unstressed), with some of the of proteins being up-regulated or down-regulated. The appearance of protein spots on the gels was evidence of osmotic-stress induced proteins obtained from maize leaf extracts through the treatment. Conceptually, each spot on the resulting 2D gel, potentially corresponds to a single protein species in the sample [32]. Our results, notably, are in agreement with the previous reports from various crops, which indicated a coordinated response to osmotic-stress [19, 33-36].
CONCLUSION
Findings from this study established that PEG affects the morphological and proteomic profiles of growing maize. Eight differentially expressed proteins were visualized and profiled, indicating that the qualitative proteomic tools used in this study were able to separate and allow for the detection of stress responsive proteins in Zea mays. Furthermore, the expressed and profiled proteins in our study provide new insights regarding the response of maize to osmotic-stress and their probable association with morphological and molecular pathways. These results may contribute to the existing knowledge, assist in providing information and creating groundwork for further identification of osmotically stressed proteins in maize that would lead to a better understanding of their roles in stress response and adaptation mechanisms. Further work, to this study would be on specific identification of each of the profiled osmotic-stress proteins through mass spectrometry, western blotting analysis, iTRAQ and in silico functional studies to possibly establish the molecular responses used by the R450w/uo2250w CML550Z. mays cultivar against osmotic-stress.
AUTHOR'S CONTRIBUTION
TBD and OR conceived the idea and designed the study; provided facilities for the study as well as supervising the experimental work. LT performed the experiments. All authors contributed to the writing, and approval of the manuscript final version.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This project was supported by the North West University, South Africa. The authors acknowledge and express their gratitude for the support.


