All published articles of this journal are available on ScienceDirect.
Measurement of the Bio-Mechanical Properties of Two Different Feeder Layer Cells
Abstract
Introduction:
We here present our findings on 2 types of feeder layers, one composed of mouse embryonic fibroblasts (MEF) and the second one of mouse skeletal myoblasts (C2Cl2) feeder cells.
Methods:
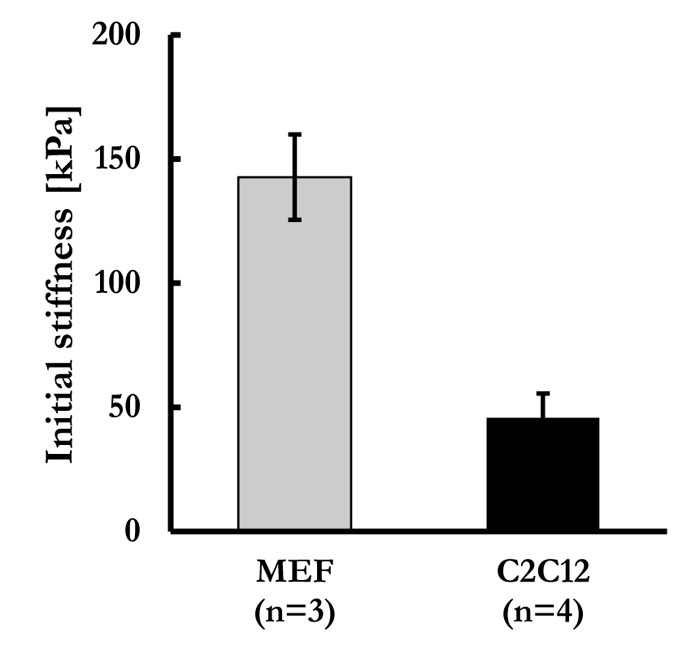
The 2 feeder layers present a dramatic variance of intrinsic stiffness (142.68 ± 17.21 KPa and 45.78 ± 9.81 KPa, respectively).
Results and Conclusion:
This information could be used for a better understanding of cells and cell microenvironment mechano-physical characteristics that are influencing stem cell commitment, in order to develop a suitable engineered tissue for cardiac and skeletal muscle repair and a bio-actuator.
1. INTRODUCTION
Muscle cells present intrinsic mechanical function, given by high energy conversion abilities of adenosine triphosphate (ATP) chemical energy [1, 2]. As other stem cells, they are able to self-repair and self-renewal [3]. For these peculiar properties muscle cells and tissues have been proposed as attractive materials to be use as “bio-actuator”, which is defined as a motor driven by living materials, i.e. biological molecules [4], living cells [5] and living tissues [6]. Moreover, muscle cells appear to be an optimal cell source for bio-actuators, because of their mechano-physical properties of being “soft” [7] and of having a micro-scale size, that allows the development of bio-microactuators. Maturation of muscle stem cells is greatly affected by the extracellular microenvironment they interact with. As other adult stem cells, they usually reside in a stem cell niche, where a complex interplay of biological and physical features maintain their multipotentiality. Main component of the extracellular context is the extracellular matrix (ECM). Studies regarding ECM structural proteins (e.g. fibronectin, laminin, collagen) and soluble molecules (e.g. hormones, growth factors) have already been the subject of intense investigation. Because of the hostile environment that cells encounter when transferred in vitro, stem cells often require the support of a feeder layer, placed between culture dishes and cells. Feeder layers are composed of mitotically inactivated cells that are still able to supplement the cells seeded on top with beneficial signals, without their own further growth or division. The most common feeder cells currently used are mouse embryonic fibroblasts (MEF) cells. Other cell types utilized for stem cell culture support are testicular stromal cells, as JK1, and embryonic mesenchymal stem cells (10T1/2) [8, 9]. Alternatively, human adult uterine endometrial cells (hUECs), human adult breast parenchymal cells (hBPCs), and human embryonic fibroblasts (hEFs) have also been used as feeder cells [10, 11]. Feeder systems have been often used in induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) cell culture [12, 13]. However, several studies demonstrated that other cell type functionality is also improved when feeder layers are used to support their growth. In particular, myoblast growth and differentiation appeared to be positively regulated by fibroblast substratum [14], with the metabolites released from cells applied as a substratum being crucial. We have previously discovered that a set of genes encoding for the expression of cytokines and chemokines are upregulated in myoblast feeder layer compared to fibroblasts feeder layer cells, and that those molecules are responsible for the stimulation of myogenic differentiation [15]. However, there is currently no clear understanding of the entire group of factors involved in the process. Influence of the extracellular microenvironment, including the impact of its physical properties, is known to be important for stem cell commitment [16, 17]. In this regard, ECM physical properties have been considered as cues affecting or directing cell fate function. Several studies demonstrated that stem cell adhesion, growth and differentiation can be regulated by physical interactions with local ECM [18-20]. Among them, substrate stiffness showed a great influence on stem cell behavior [20, 21] and several groups worked on recreating the specific tissue mechanical microenvironment in vitro, using both synthetic and natural materials [22]. In particular, the behavior of muscle stem cells appeared to be highly sensitive to substrate stiffness [23-25].
A wide range of materials is currently being investigated with the purpose of recreating an in vitro system able to decouple the elasticity parameter for studying the direct influence of mechanical properties on muscle cells. We previously demonstrated that films composed of a mixture of 2-branched and 4-branched poly-ε-caprolactone (PCL), showing no variation in substrate nanotopography and having diverse stiffness values, could affect cardiac and skeletal muscle cells behavior [26, 27]. In addition, when biologically related cells (rat skeletal myoblasts) and non-related cells (mouse embryonic fibroblasts and normal human dermal fibroblasts) were used as feeder layers in between PCL films and C2Cl2, a significant difference could be found in the percentage of differentiated myotubes in the two systems. These results suggest that different cell types have defined response to the microenvironment mechanical characteristics and that those feeder cells have intrinsic elasticity affecting C2Cl2 myogenesis [27].
The most common technique used to detect cell stiffness is through the Atomic Force Microscope (AFM). Many studies have demonstrated the specificity of AFM method to determine single cell stiffness [28-31]. Other methods include tensile test of cells seeded on gels, micropipette aspiration [32], optical trap detection [33-35] and magnetic twisting cytometry [36]. We here propose a novel measurement technique for the bio-mechanical properties, such as adhesion force, stiffness and beating force of living materials (cell sheet, 3D tissues and native tissue) for the evaluation of bio-actuators and for medical application [7, 37]. Peculiarity of this method is expressed by the ability of measuring the mechanical property of an integrated multiple group of cells and of the ECM. In fact, other techniques have already proved that cells derived from various parts of the body display different mechanical properties, however, no other stiffness measurement systems known was able to quantify these characteristics. In order to confirm our hypothesis of different feeder layers mechanical properties influencing C2Cl2 myogenic differentiation, we here investigated their mechanical properties, considering cell sheets developed as feeder cells layers. Thus, we analyzed the stiffness of the feeder layers made by two cell types: MEF and C2Cl2.
2. MATERIALS AND METHODS
2.1. Cell Culture
Skeletal myoblasts behavior was studied using mouse cell line C2Cl2 (CRL-1772; ATTC, Rockville, Maryland, USA). Mouse embryonic fibroblasts (MEF) were purchased from Applied stem cell (CF-1 MEF; California, USA). Both cell types were cultured in Dulbecco's Modified medium with 4.5 g/L Glucose (DMEM; Nacalai Tesque Inc., Kyoto, Japan) supplemented by 10% Fetal Bovine Serum (FBS; Life Technologies, Gaithersburg, Maryland, USA) and 1% penicillin/streptomycin solution at 5000 µg/mL (Invitrogen), in a condition of 37 °C in 5% CO2. Every 2 days, cells were treated with 0.25% trypsin/1 mM ethylenediaminetetraacetic acid (EDTA, Nacalai Tesque Inc.) and transferred to a new tissue culture polystyrene (TCPS; Iwaki Co., Tokyo, Japan). When required, C2C12 and MEF were treated with Mitomycin C.
2.2. Feeder Layer Preparation
C2Cl2 and MEF feeder layers were obtained by treating confluent cell layers with Mitomycin C. First, C2Cl2 and MEF were seeded on TCPS with a confluence of 1.0 × 106 cells/cm2 C2Cl2 and 2.0 × 106 cells/cm2, respectively. Different cell number was used to overcome C2C12 and MEF cell doubling time difference (12h for MEF versus 24h in C2Cl2 [38, 39].
After 24 h, cells were treated with 10 µg/mL Mitomycin C (Sigma-Aldrich Co., St. Louis, USA) for 2 h, trypsinized, resuspended in complete medium and plated for use in 0.1% gelatin-coated plate (Sigma-Aldrich Co.).
2.3. Measurement of Cell Mechanical Properties: Cell Sheets Preparation and Tensile Test
C2Cl2 and MEF cells were used to prepare two different types of cell sheets. First, 60 mm polystyrene dishes coated with temperature-responsive polymer poly (N-isopropylacrilamide) (PIPAAm) (UpCell, CellSeed, Tokyo, Japan), were pre-incubated with 2 mL FBS each. After 6 hours, FBS was removed and cells detached from normal culture dishes were seeded on PIPAAm and cultured at 37 °C and in 5% CO2 for 3 days. Because C2C12 doubling time is faster than MEF cells (12h for MEF versus 24h in C2C12 [38, 39], the 2 types of cells were seeded at different densities: 1.0 × 106 C2Cl2 cells were seeded for myoblast cell sheet formation, and 2.0 × 106 MEF were plated on PIPAAm, in order to obtain same cell density. After 3 days, tensile testing was performed following the procedure previously described [40]. The strain speed was adjusted to 0.5% per sec (0.1mm/s), instead of 1% per sec (0.2 mm/s). A lower speed is believed to give less influence to the measurement of real stiffness value attributed to the cell sheets, through this methodology [41]. Additionally, the thickness of cell sheet was assumed as 10 μm.
3. RESULTS
We previously demonstrated that biological related and non-related feeder cells affected in different ways myogenic differentiation of C2Cl2 myoblasts seeded on top [27]. We also showed that feeder layers formed by cells of different or same species of cells seeded on top, did not influence their ability to support cell growth [15, 27].
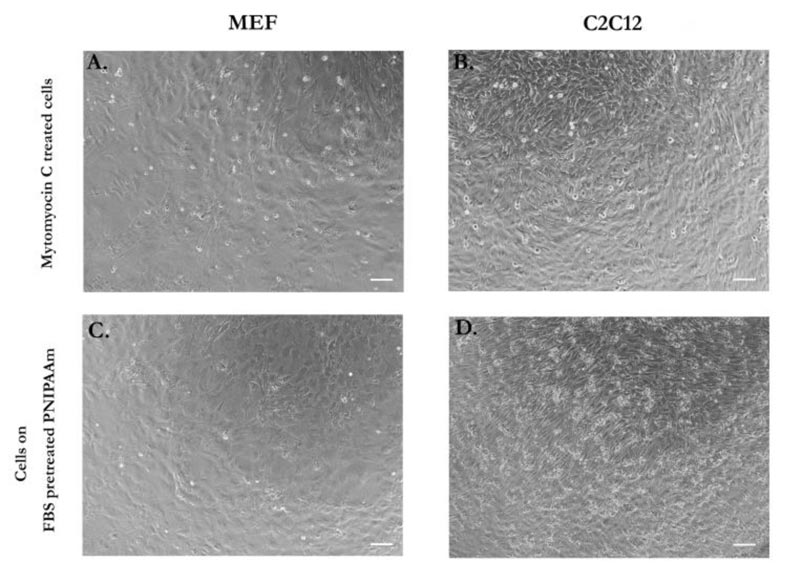
In order to analyze if mechanical properties, such as stiffness, could differ in feeder layers made by the unrelated cell types, we first assumed that cells in a feeder layer could be in a similar condition to cell sheets generated by temperature-responsive technology. The assumption was made on the principle that in both systems, cell proliferation is inhibited. In case of feeder layer conditions, cell proliferation is obstructed by a phenomenon known as contact inhibition, where cells coming in contact with other cells stop proliferating in in vitro conditions. Contact inhibition is in fact, a powerful anticancer mechanism of healthy cells that is lost in cancer cells [42]. In feeder systems, mitotic activity was blocked by the treatment with 10 µg/mL Mitomycin C. In cells seeded on PIPAAm temperature-responsive dishes, cell-cell contact and cell detachment from the substrate before tensile test inhibited cell ability to grow. Cell morphology of feeder layers and cells cultured on PIPAAm dishes was compared to confirm our hypothesis. Both inactivated fibroblasts (Fig. 1A) and in cell sheet mode (Fig. 1C), showed same cell morphology. Myoblasts feeder cells treated with Mytomycin C (Fig. 1B) and seeded on temperature-responsive dishes (Fig. 1D) showed similar features after three days of culture. Cells that require feeder systems, such as iPSCs and ESCs, can sense the overall feeder layer mechanical properties, rather than individual cell elasticity that compose the feeder layer. In order to measure such structure, tensile mechanical test was chosen as a method of measurement over AFM technique, from which single cell or different parts of cell stiffness can be assessed.
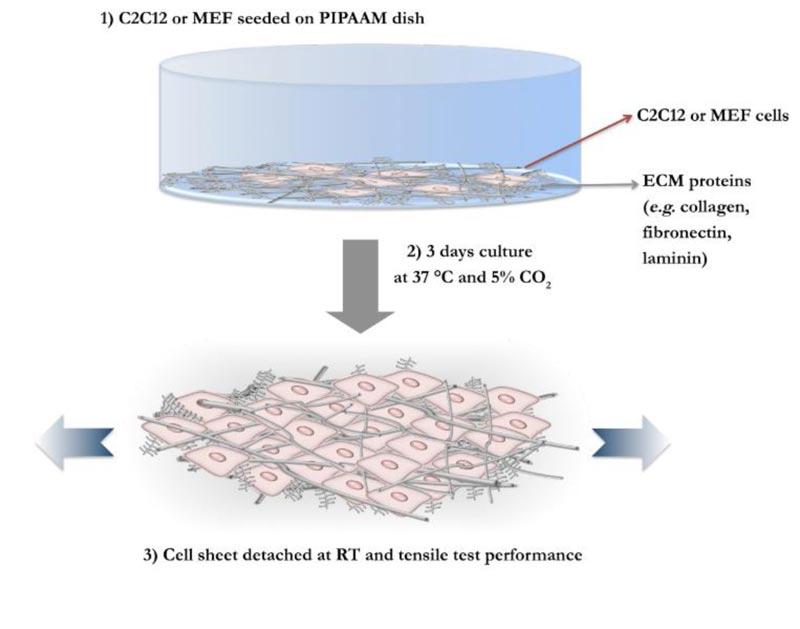
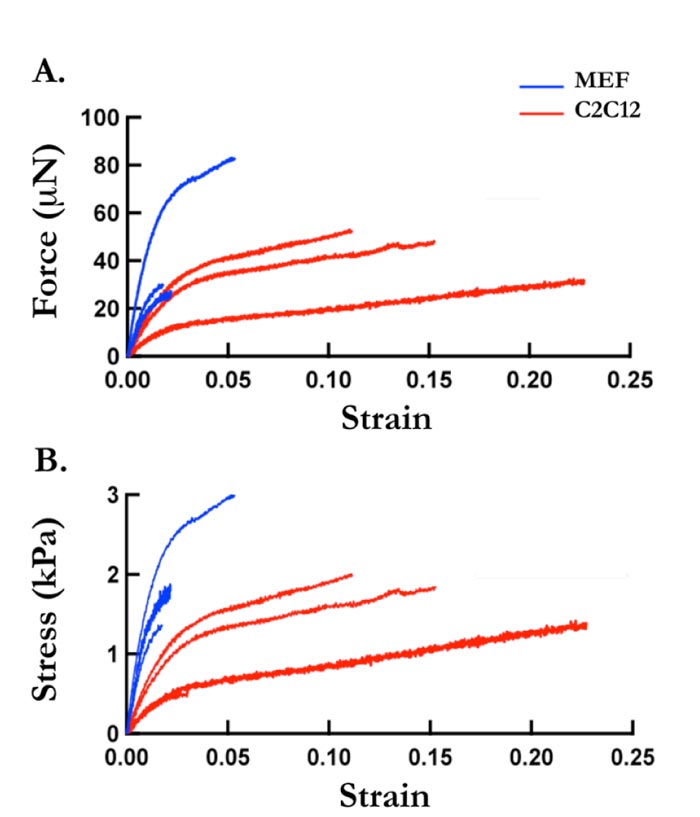
Thus, feeder cell elastic properties were measured. We previously successfully developed a tensile testing technique that permits measurement of cells sheet stiffness, where detached cell sheets were tested for their tensile mechanical characteristics. A schematic representation of the adopted system is given in Fig. (2). In this study, MEF cell sheets and C2Cl2 cell sheets elasticity were measured through tensile testing technique, as previously developed (Supp. video 1). In Fig. (3), force-strain and stress-strain curves of MEF (blue lines) and C2Cl2 cell sheets (red lines) show the stride by which each cell sheet was stretched during the tensile test. The Young’s Modulus values obtained by each experiment were grouped and average values for MEF cell sheets and C2Cl2 were given (Fig. 4). As expected, when the two cell sheet types stiffness values were compared between each other, we observed that MEF cell sheets had higher stiffness values (142.68 ± 17.21 KPa) as compared to cell sheets made by C2Cl2 (45.78 ± 9.81 KPa).
4. DISCUSSION
Bio-mechanical properties of cells and tissues are important parameters for their application in the medical field and the bio-actuator. Endogenous forces produced by cells and their extracellular matrix (ECM), as well as contacts with neighboring cells, are believed to influence tissue mechanical environment and cell fate and function [18, 43].
Currently, the most used approach for the evaluation of cell stiffness is the AFM technique [29, 44]. This system is very efficient in determining single cells stiffness and elastic property differences within one cell accurately. The accuracy of the method resulted in the ability of telling differences in the local Young’s Modulus within the same cell, depending on the specific area of the cell where the measurement was performed [45]. However, this method was not able to give bulk information about a group of cells considered as one system. Moreover, even though independent research groups were able to evaluate cell stiffness, still large discordance in the absolute stiffness values persists, suggesting that there is no standardization for cell stiffness evaluation at present. The method developed from our group allowed us to measure the Young’s Modulus of a multicellular structure (an artificial tissue), as a feeder layer or a cell sheet. This method does not suffer from the bias due to random local measurements and directly averages the stiffness of the in vitro cell construct [40]. Additionally, rather than giving information on the local stiffness, this method also considers the contribution of cell-cell interaction and the resistance due to the presence of the extracellular matrix. Furthermore, the peculiarity of performing tensile test of cells surrounded by culture medium, overcomes the issue of inaccuracy due to cell tendency to dry, a phenomenon occurring in other measurement methods [40].
In this study, fibroblasts feeder layers showed a significantly higher stiffness value as compared to C2C12 feeder cells. Fibroblasts are known to play a critical role in the formation of scar by massively producing ECM proteins as collagen, fibronectin and elastins [46], and building a stiff replacement tissue. This result strengthens our hypothesis that our method could detect ECM and cell-cell contact influence in substrate elasticity together with intrinsic single cell mechanical feature. In fact, it was already demonstrated that cell sheet mechanics is directed not only by focal adhesion traction forces but also by the forces transmitted by intercellular junctions to neighboring cells. On the contrary, C2C12 myoblasts cells are committed to generate contractile functional units for muscle tissues and thus need to be highly compliant. Hence, our findings are in accordance with the knowledge of in vivo mechanical properties of fibroblasts and myoblast cells. The stiffness values obtained appeared to be higher than values obtained by other methods, as AFM measurements, where skeletal myoblasts appeared to have a Young’s Modulus of 12-15 KPa and fibroblasts around 17 KPa [29, 47-49]. This result can be explained by the difference between the 2 measurement methods of tensile test and indentation measurements (i.e. AFM). Additionally, in our system, cell sheet fixing before tensile test performance has to be considered in the measurement force for the initial cytoskeleton tension and its reorganization [40]. These two types of forces have not been quantified so far, but clear differences between mechanical properties of single cells and cell sheets could be detected when cells were analyzed for their response to substrate stiffness changes [50, 51].
During the tensile test performed in this study, a difference in the elastic and plastic deformation of MEF and C2Cl2 cell sheets could be detected. MEF system showed higher and faster elastic deformation compared to C2Cl2 after the first 0.02 sec of stretching (Fig. 3).
CONCLUSION
Conditions of muscle cells and tissues like differentiation represent important parameters for applying these cells to the medical field and the bio-actuator.
Many studies have already shown that mechanical and several biological factors are involved in muscle cell differentiation. In this study, we demonstrated that cells and their microenvironment biomechanical properties, such as stiffness, differ significantly among specific cell types. Many techniques have already been developed for measuring the mechanical properties of single cells. However, these methods were not able to give bulk information about a group of cells considered as one system. Besides, the technique developed by our group for measuring feeder cell mechanical properties, performing tensile tests of cell sheets generated by different cell types, provided additional information in comparison with other stiffness measurement methods. Fibroblast feeder layers appeared to have higher stiffness as compared to myoblast feeder cells. This result could explain our previous study showing that myoblasts feeder cells could improve myogenic differentiation of C2Cl2 seeded on top. The molecular basis of the mechanotransduction process leading to these results, meaning how mechanical properties are converted to biochemical signals during these co-culture experiments, are still unclear and further investigations are required.
AUTHOR'S CONTRIBUTION
S.R., K.U., K.M. and A.T. conceived and designed the experiments; S.R. and K.U. performed the experiments; S.R. and K.U. analyzed the data; A.T. and G.F contributed to the interpretation of the results and supervised the findings of this work. S.R. and K.U. wrote the initial draft of the manuscript and all authors have contributed to data interpretation and approved the final version of the manuscript.
NOTES ON CONTRIBUTORS
Sara Romanazzo obtained both BS and a MS in Molecular and Cellular Biology at University of Rome Tor Vergata (Rome, Italy) in 2011. She completed a PhD in Nanoscience and Nanoengineering at Waseda University (Tokyo, Japan) in 2015 and subsequently started a postdoctoral position in a joint program between the Trinity Centre for Bioengineering, Trinity College Dublin (Ireland) and The Royal College of Surgeons in Ireland. From 2018 she is a postdoctoral researcher at the University of New South Wales, Australia, where her research is focused on bone and vascular tissue engineering, including 3D printing techniques for tissue regenerative therapies.
Kaoru Uesugi received his BS degree in electronic engineering from Shibaura Institute of Technology, Tokyo, Japan, in 2008. He received his MS degree in Mechanical Systems Engineering from Tokyo University of Agriculture and Technology, Tokyo, Japan, in 2010. He also received his PhD in Mechanical Engineering from Osaka University, Osaka, Japan, in 2014. He is currently a research assistant professor of Osaka University. His current research interests include biomedical sensors and transducers, bio-MEMS, biomechanics and mechanobiology
Akiyoshi Taniguchi obtained his PhD at Toho University, Japan in 1999. He is currently professor at the Graduate school of advanced science and engineering at Waseda University, Tokyo, Japan and leader of the “Cell-Materials interaction” group at the National Institute for Materials Science.
Giancarlo Forte obtained his MS in Molecular and Cellular Biology (2000) and his PhD in Experimental Pathophysiology (2005) at the Medical School of the University of Rome Tor Vergata, Rome, Italy. He was then postdoctoral fellow at the Italian Institute for Cardiovascular Research (INRC), and senior researcher at the National Institute for Materials Science, Tsukuba, Japan from 2010. From 2014, he is group leader at the Center for Translational Medicine (CTM), and vice-chair of the International Clinical Research Center (ICRC) of St. Anne’s University Hospital, Brno, Czech Republic.
Keisuke Morishima received his BS, MS and PhD from Nagoya University, Aichi, Japan, in 1993, 1995, and 1998 respectively. From 2005, he was an associate professor at Tokyo University of Agriculture and Technology, Tokyo, Japan. From 2012, he is currently a professor at Osaka University, Osaka, Japan. His current research interests include bioMEMS, bioactuator, and biomedical wet robotics.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The present work was partly supported by the European Regional Development Fund - Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123) to Giancarlo Forte.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


