All published articles of this journal are available on ScienceDirect.
Does Fried Street Food (Kokor) affect Lipid Profiles and bodyweight? The Finding from Swiss Albino Mice
Abstract
Background:
Foods fried in palm oil on the streets change the oil composition and produce toxic byproducts. Even though the health implications of fried street food are not fully understood, Ethiopians frequently consume these items. Therefore, this study evaluated the impact of street kokor fried in palm oil on mice's lipid profiles and body weight.
Methods:
The experiment involved 32 Swiss Albino male and female mice, which were randomly separated into four groups with equal male and female subgroups. The experimental groups, Group II, Group III, and Group IV, got 10%, 20%, and 30% of the daily food intake, respectively. In contrast, the control group (Group-I) received only pellets and a vehicle (water). The mice were killed at the end of the sixth weeks after recieving a diethyl ether anesthetic. Once their blood was drawn through a heart puncture, lipid profile tests were performed on it.
Results:
In this experiment, the amount of street kokor cooked in palm oil had a significant impact on the mice' body weight [F (3, 24) = 13.841, p = 0.001] and all of the mice in the experimental groups had significantly lower body weights than the mice in Group I (the control group) (P 0.05). Similarly, the dose of palm oil fried street kokor had a significant effect on serum triglyceride (TG) [F (3, 24) = 17.72, p = 0.001], serum low-density lipoprotein (LDL) [F (3, 24) = 90.344, p = 0.001], serum high-density lipoprotein (HDL) [F (3, 24) = 25.38, p = 0.001] and serum total cholesterol (TC) level of the mice [F (3, 24) = 257.480, p = 0.001]. The experimental group mice's lipid profiles, except serum HDL level, were increased significantly compared with the control group mice (P < 0.05).
Conclusion:
Mice's body weight fell and serum lipid profiles were affected by palm oil-fried street kokor. This study found that palm oil-fried street kokor dramatically decreased mice's body weight. Furthermore, ingesting kokor cooked in palm oil significantly and proportionally elevated mice's serum lipid profiles (TG, LDL, and TC), but it also dramatically and inversely decreased HDL levels.
1. INTRODUCTION
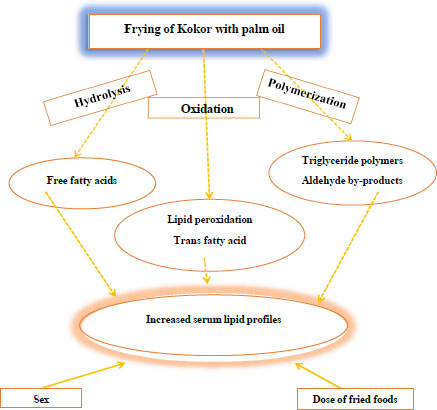
One category of ready-to-eat street food in various hygienic conditions and characteristics is oil-fried food [1, 2]. They are fried in hot oil to give them a distinct flavor, texture, and appealing look [3]. The physical and chemical characteristics of oils alter when they are used to cook food, especially when it is done repeatedly. Some of the changes that occur when oils are cooked include hydrolysis, oxidation, and polymerization [4].
Oil and fat hydrolysis release free fatty acids into the bloodstream, which increases lipoprotein levels and results in atherosclerosis [5]. Oil oxidation during frying results in numerous metabolic changes in the body. Free radicals and trans fatty acids are produced as a result of lipid peroxidation [6, 7], where the presence of trans double bonds causes the fatty acyl chains to align straight, making fats more viscous (solid) at room temperature [8]. Total polar compounds (TPC), which are produced during frying by polymerization processes, result in lipid deposition, cytotoxicity, and oxidative stress [9].
Many communities' main source of food and money is fried street food. Despite these benefits, they are marketed in filthy settings, supplied in dangerous containers, and of inferior quality [10]. Street food snacks in India can have up to 69% saturated fats and 30% trans fats in their total fatty acid composition [11]. Trans-fatty acid chains may disturb membrane phospholipids and affect membrane fluidity. They negatively impact cell activities and functioning in a variety of ways [12, 13]. Additionally, they raise levels of low-density lipoprotein (LDL), the plasma LDL/HDL ratio, and the ratio of total cholesterol to HDL (TC/HDL), all of which are detrimental to cardiovascular health [14] and change body weight [15, 16]. Atherosclerosis, a condition marked by the buildup of lipids and inflammatory cells in the inner lining of major arteries, is brought on by high concentrations of the aforementioned compounds in the blood [17] (Fig. 1).
Although Africa has not yet seen the effects of nutritionally related non-communicable diseases (N-RNCD), as calorie availability rises in the years to come, the burden of N-RNCD disorders may increase. N-RNCDs (diabetes, cardiovascular disease, and others) cause almost one-third of all fatalities in Africa, despite scant data sources on their true prevalence [18].
Ethiopia's diet and feeding practices vary because Ethiopia is a country with a diverse cultural population. However, there hasn't been much research done on how these various foods and feeding habits affect population. Therefore, we looked at mice's lipid profiles and body weight after eating street kokor fried in palm oil.
2. MATERIALS AND METHODS
2.1. Experimental Mice and the Study Design
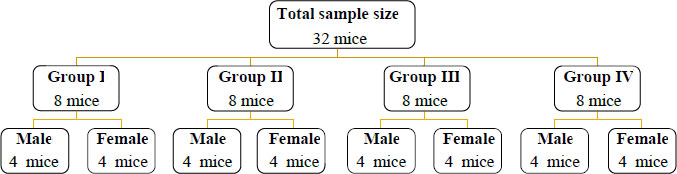
The investigation was conducted at Jimma University's Veterinary Medicine Laboratory. The experiment used thirty-two (32) Swiss Albino mice, ranging in age from 10 to 12 weeks. They lived in similar environments, experienced 12-hour light/dark cycles, and accessed pellets and distilled water at libithum. The mice were acclimated for one week before the experiment.. After that, they were sorted into four (4) groups of eight mice each, and again into two (2) subclasses as male and female sets in various cages. The National Institutes of Health's recommendations serve as the basis for all procedures [19] and the Arrive Guidelines 2.0 [20] (Fig. 2).
2.2. Kokor Preparation and Administration
The ingredients for the kokor (wheat flour (3 kg), palm oil (1 L), and yeast) were purchased from neighborhood grocers in Jimma city, southwest Ethiopia. The kokor was prepared by food vendors on the streets of Jimma town following local customs. It was then broken into small pieces, allowed to dry in the sun for three days, and then pounded with a mortar and pestle until it was powder. Up until administration, the powder was kept in a tidy, dry glass container. Standard pellets and water were provided to group one andthe control group, as needed. According to their body weight, the experimental groups (groups two, three, and four) received 10%, 20%, and 30% of their minimal daily dietary needs in the form of fried kokor, respectively [21]. Each mouse's daily dosage was divided into two equal doses and given twice daily using a 24-gauge oral gavage needle for six weeks. This was after the kokor was dissolved in distilled water at 300 mg/ml [22].
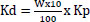 |
Keys
Kd = Dosage of kokor given to each mouse in one day (g)
W = Body weight of each mouse (g)
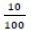 = Daily food needs of mice relative to their body weight [23]
= Daily food needs of mice relative to their body weight [23]
Kp = Proportion of kokor given to each mouse in a specific group (%).
2.3. Data Collection Procedure
Mouse body weight was measured once a week. By the end of the sixth week, they had been denied food for 12 hours and died from diethyl ether asphyxiation [24]. Following a heart puncture, two to three milliliters of blood were drawn and stored in a serum tube at -200C for 30 minutes before centrifugation.. The serum was then separated by centrifugation at 3000 revolutions per minute for 10 minutes at room temperature. The lipid profiles (TG, LDL, HDL, and TC) were assessed.
2.4. Study Variables
The study variables included body weight, sex, blood lipid profiles (TG, LDL, HDL, and TC), and kokor dose..
2.5. Data Quality Assurance
Pre-analytical, analytical, and post-analytical safeguards were considered prior to analysis, and the equipment was calibrated using established standards. Using the ABX Pentra 400 Clinical Chemistry auto-analyzer (HORRIBA ABX SAS, China) in accordance with the machine's instructions, qualified specialists evaluated the serum lipid profile values (TG, HD, LDL, and TC).
2.6. Data Processing and Analysis
The data was entered into Epi Data software version 3.1 after examination and cleaning. The data was then exported to SPSS software version 25.0 for analysis. The analysis findings were presented in table and reported as mean and SD. Two-way ANOVA (Tukey) was used to examine the data, and results with a P value of less than 0.05 were deemed statistically significant.
2.7. Ethical Considerations
The Jimma University Institutional Ethical Review Committee issued an ethical clearance letter with reference number IHRPGD/680/2019. A cooperation letter from the Agriculture and Veterinary Medicine College was obtained. During this study, the WMA Declaration of Helsinki governing biomedical research involving animal experimentation was followed.
3. RESULTS
3.1. Bodyweight of Mice
Genetic, physiological, and behavioral factors affect body weight. One of the variables influencing body weight is improper nutritional consumption [25]. In our investigation, it was established that the kokor dosage and the mice' gender did not substantially interact with each other's effects on body weight [F (3, 24) = 0.299, p = 0.826]. Accordingly, there was no discernible difference in the body weight of the mice based on their gender [F (1, 24) = 2.689, p = 0.114]. However, the kokor significantly altered the mice' body weight [F (3, 24) = 13.841, p = 0.001] (Table 1).
3.2. Serum Lipid Profiles of Mice
For predicting cardiovascular risk, serum lipid profiles are evaluated and have four fundamental components (TG, LDL, HDL, and TC) [26]. The serum lipid profiles of these study mice were analyzed Table 2.
3.2.1. Serum TG
This study found that mice's sex and kokor dosage had no discernible effects on TG levels [F (3, 24) = 0.098, p = 0.961]. In a similar vein, the mice' TG levels were not significantly influenced by their gender [F (1, 24) = 1.283, p = 0.268]. However, the amount of kokor administered to the mice had a significant impact on their TG levels [F (3, 24) = 17.72, p = 0.001] (Table 2).
| Groups | Final Body Weight (g) | |
|---|---|---|
| Male | Female | |
| Group one | 42.98±4.21a | 41.38±1.92a |
| Group two | 38.70±1.66b | 36.78±1.60b |
| Group three | 36.73±1.45b | 35.88±1.28b |
| Group four | 37.40±0.72b | 37.18±0.56b |
| Variables | Sex of Mice | Group One | Group Two | Group Three | Group Four |
|---|---|---|---|---|---|
| TG (mg/dl) | Male | 128.54±6.98a | 138.27±8.89b | 146.24±7.49bc | 154.64±5.47c |
| Female | 123.45±11.93a | 135.84±8.25b | 145.12±5.93bc | 150.11±4.61c | |
| LDL (mg/dl) | Male | 19.00±2.15a | 22.10±2.65b | 32.29±2.08c | 34.18±1.61c |
| Female | 20.60±2.24a | 24.79±1.69b | 32.78±2.30c | 34.68±2.00c | |
| HDL (mg/dl) | Male | 42.46±0.72a | 38.69±3.12b | 36.06±1.76c | 35.33±0.91c |
| Female | 41.35±1.83a | 37.73±2.69b | 34.81±0.50c | 34.60±0.81c | |
| TC (mg/dl) | Male | 93.40±1.32a | 100.17±2.60b | 110.46±1.55c | 114.01±0.33d |
| Female | 92.67±1.10a | 98.02±2.82b | 109.98±1.55c | 113.38±0.69d |
3.2.2. Serum LDL
Fresh palm oil lowers blood LDL levels, but as it oxidizes, it alters plasma lipids, free fatty acids, phospholipids, and cerebrosides [27]. The dosage of the kokor and the mice' gender in the current investigation had no discernible effects on the serum LDL levels of the mice [F (3, 24) = 0.505, p = 0.683]. Similarly, the mice' gender had no appreciable impact on their serum LDL levels [F (1, 24) = 3.164, p = 0.088]. However, the dose of kokor had a substantial impact on the mice' blood LDL levels [F (3, 24) = 90.344, p = 0.001] (Table 2).
3.2.3. Serum HDL
Foods with higher energy and fat content but lower in vegetable and grains reduce serum HDL [28]. The dosage of the kokor and the mice' gender in the current investigation had no discernible effects on the mice's HDL levels [F (3, 24) = 0.031, p = 0.992]. Similarly, mice's gender had no appreciable impact on HDL levels [F (1, 24) = 2.564, p = 0.122]. But the mice' HDL levels were significantly impacted by the kokor dosage [F (3, 24) = 25.38, p = 0.001] (Table 2).
3.2.4. Serum TC
When consumed in an oxidized condition, palm oil exhibits hypercholesteremic properties because saturated fatty acids make up half of its composition [27]. The amount of kokor given to mice and their gender had no discernible effects on their TC levels in this investigation [F (3, 24) = 0.407, p = 0.750]. Similarly, neither mice's sex nor TC levels were significantly different [F (1, 24) = 2.889, p = 0.102]. But the amount of kokor administered to the mice had a significant impact on their TC level [F (3, 24) = 257.480, p = 0.001] (Table 2).
4. DISCUSSION
In our investigation, the final body weight of the mice in the intervention groups was considerably lower than that of the mice in Group 1 (the control group) (Table 1). This study confirms research by A. Falade and colleagues on rats in Nigeria and by Idris and colleagues on rabbits in Malaysia. Both showed that participants who consumed palm oil had lower weights and BMIs [16, 29] but didn’t agree with Morshed and colleagues' findings on rabbits in Bangladesh [30]. The physiological variations between the two experimental animals could be the cause of the disparity (mice and rabbits). Although it was not statistically significant, the experimental group's mice body weight fell as the kokor dose increased. This result is consistent with the observation made on mice by Boniface M.N. and his associates. They found that as the dose of thermally oxidized oil increased, mice' body weight declined [31].
The loss of body weight in mice may be caused by cellular death in their bodies due to the oxidized chemicals created during oil frying [32]. The other suggested reason for weight loss is related to the substantial lipid polymers created during palm oil frying. These polymers are less absorbable and decrease the absorption of fat-soluble nutrients from the stomach, which leads to a decrease in their body weight [33].
In comparison to the control group (Mice from Group-I), the TG level of the interventional group mice was considerably higher (P < 0.05). Additionally, there was a significant difference in TG levels between Group 2 and Group 4 mice (P < 0.05) between the two groups of mice. This study agrees with Morshed and his colleagues. They demonstrated that repeatedly heating palm oil raises rabbit TG levels [30]. The increase in free fatty acids produced during the frying process may be connected to the increase in TG found in the serum of experimental mice [34].
The LDL level of interventional group mice was significantly increased than group one mice (P < 0.05) and the LDL level of group four and group three mice was higher than group two mice's LDL level significantly (P < 0.05). These findings are consistent with other findings of previous studies by different scholars in different times and places that describe repeatedly heated palm oil increases serum LDL levels [16, 29]. The increment of LDL in the serum of the mice could be related to the high content of saturated fatty acids and trans fatty acids in the palm oil fried kokor [28].
The HDL levels of the interventional group mice were significantly less than the HDL levels of group one mice (P 0.05), and the HDL levels of group four and three animals were significantly less than the HDL levels of group two mice. The outcome of this investigation confirms the conclusions of earlier research by Ayodeji and Ilyas alongside their associates, which indicated that oxidized palm oil lowers serum HDL levels [29, 35]. The trans fatty acids produced during kokor oil-frying could be the cause of mice's lower HDL levels [36]. However, other previously reviewed research shows that a modest increase in HDL was seen in experimental groups fed oxidized palm oil. This may be because trans fatty acid levels were low in these trials, as trans fatty acid synthesis depends on a variety of circumstances [37].
CONCLUSION
Mice's body weight fell and serum lipid profiles were affected by palm oil-cooked street kokor. This study found that palm oil-fried street kokor dramatically decreased mice's body weight. Furthermore, ingesting kokor fried in palm oil significantly and proportionally elevated mice's serum lipid profiles (TG, LDL, and TC), but it also dramatically and inversely decreased HDL levels.
LIST OF ABBREVIATIONS
| HDL | = High-density lipoprotein |
| LDL | = Lowdensity lipoprotein |
| TG | = Triglyceride |
| TC | = Total cholesterol |
AUTHORS’ CONTRIBUTIONS
HA and TY conceptualized the research idea and wrote the proposal. They edited and reviewed the proposal, the laboratory set up and conducted the experiment, as well as analyzed the statistical analysis. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Jimma University Institutional Ethical Review Committee issued an ethical clearance letter with reference number IHRPGD/680/2019.
HUMAN AND ANIMAL RIGHTS
A cooperation letter from the agriculture and veterinary medicine college was obtained. During this study, the WMA declaration of helsinki governing biomedical research involving animal experimentation was followed.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIAL
The data supporting the findings of the article is available in the [Jimma University Open Access Institutional Repository] at [http://10.140.5.162//handle/123456789/2935], reference number [517].
FUNDING
This study was funded by Mizan-Tepi University. The funder was not involved in the manuscript writing, editing approval or publication decision.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGMENTS
The authors acknowledge Jimma University's Veterinary Medicine Laboratory staff for their collaboration and support during the study.


