All published articles of this journal are available on ScienceDirect.
Antimicrobials Misuse/Overuse: Adverse Effect, Mechanism, Challenges and Strategies to Combat Resistance
Abstract
Overuse and misuse of antibiotics are the first risk factors for the development of antibiotics resistance. Inadequate professional competence of health care physicians might worsen the complications associated with antibiotics resistance. Antibiotic resistance is a global issue; however, the epicenter of this misfortune is Asian regions due to the easy accessibility of the strongest antibiotics without prescriptions or diagnoses. High effectiveness and easy accessibility of antibiotics lead to overuse/misuse and encouraging bacteria to develop the resistance. The over-usage and mis-usage of antibiotics are antibiotic abuse, which increase the potentially serious impact on human health. Bestowing to WHO guidelines, the resistance has led to spread worldwide and classifying resistance is a serious health problem. Furthermore, resistance claims uncertainty to predict the future. This review summarizes the major antibiotics involved in drug resistance, mechanism, prescribed dosage with a disease condition, proposed policies and guidelines to combat antibiotic resistance associated problems.
1. INTRODUCTION
Antimicrobial Resistance (AMR) has emerged as a major risk to public health estimated to cause 1 crore deaths annually by 2050 [1]. Annually, more than 50,000 newborns die from sepsis due to resistance against first-line antibiotics [1, 2]. The overuse and misuse of antibiotics are vital issues contributing to the development of antibiotic resistance, causing potentially serious effects on human health [3]. Several countries are facing the emergence of drug-resistant bacteria, which are completely resistant to available antibiotics. Most of the countries are preparing a country-specific action plan for the management of AMR globally, according to WHO guidelines [1, 2]. Asian countries have the largest burdens of drug-resistant strains worldwide [4]. Furthermore, India is one of the major consumers of antibiotics in the world and their overuse and misuse are rambling [4]. Besides, India is at one of the top rates of resistance against antimicrobials that are used for the treatment of humans and farm animals [5]. Currently, antibiotics are also utilizing against the treatment of several species of fungi and protozoans infections, but the impact of these is very toxic to humans and animals [6]. Antibiotics' effectiveness and easy access can lead to overuse or misuse prompting to develop resistance in microorganisms. In addition, inappropriate antibiotic dosages have also contributed to the emergence of antibiotic-resistant bacterial strains [7]. Unappropriated practices of antibiotics have long been believed to fuel AMR, but newer research showed that simply lowering consumption is not enough [8]. Significant problems are arising due to the development of antibiotic-resistant strains of bacteria and further creation of multidrug-resistant bacterial spp [9]. The multidrug-resistant bacterial spp., having resistance to multiple antibiotics, can cause life-threatening infections [10]. For example, overuse of fluoroquinolone and other associated antibiotics can also lead to such kind of antibiotic resistance in bacteria [11]. Developments of superbugs are the result of such kinds of antibiotic associated evils [12]. In addition, few antibiotics have been associated with adverse side effects, including mild to very serious symptoms that depend upon treated microbial sps and individual patients [13]. The antibiotics can sometimes alter the host microbiota and lead to chronic infection and inflammation [14]. Usually, antibiotics side-effects are fever, nausea and major allergic reactions, including photo-dermatitis and anaphylaxis. The common side-effect of antibiotics is diarrhea, due to the misbalancing of the intestinal flora species or overgrowth of pathogenic bacteria [15]. Additional side-effects included the interaction with other drugs, such as the elevated risk of damage of tendon when quinolone antibiotic is given with a corticosteroid [16]. The overuse of fluoroquinolone and other antibiotics leads to antibiotic resistance in bacteria, which might inhibit the treatment of antibiotic-resistant infections [16]. The article summarizes the adverse effect of antibiotics, mechanisms of resistance, challenges and future strategies to combat antimicrobial resistance to manage the health care system.
2. ADVERSE EFFECTS OF ANTIBIOTICS
A side effect of antibiotics is a reaction that occurs in the patients, along with the therapeutic action [17]. If the dosage of antibiotics is used appropriately, antibiotics are relatively safe, with very few side effects. However, these side effects can range from mild allergic reactions to severe adverse, which may be extremely variable from patient to patient and from antibiotic to antibiotic [18]. Moreover, many antibiotics may interfere with the individual patient’s ability to tolerate and complete the course [19]. Fewer patients having any previous allergic reaction to any kind of antibiotics are not recommended for the same. The allergic reactions may include symptoms like a skin rash and allergic reactions, called anaphylaxis, which can lead to difficulties in breath and swelling of the lips or tongue and face. Here, the common side-effects of major antibiotics are given below [20].
(1) Penicillin: Rash, diarrhea, abdominal pain, nausea/ vomiting, drug fever, hypersensitivity (allergic) reactions, etc. [15, 21].
(2) Cephalosporin: Diarrhea, nausea/vomiting, hypersensitivity reactions, serum sickness, etc. [22].
(3) Aminoglycosides: Nausea/vomiting, nystagmus, etc. [23].
(4) Erythromycin, azithromycin, clarithromycin: abdominal pain, diarrhea, anorexia, nausea/vomiting, taste alterations, etc. [24].
(5) Tetracycline: Nausea/vomiting, diarrhea, anorexia, abdominal pain, tooth discoloration in children < 8 years, liver toxicity, etc. [25].
(6) Quinolones ciprofloxacin (cipro), levofloxacin (levaquin), moxifloxacin (avelox), ofloxacin (floxin): nausea/vomiting, diarrhea, abdominal pain, headache, lethargy, insomnia, etc. [26].
(7) The environments, especially the water bodies, have also reported the presence of resistant organisms or their genes. Specific socio-economic and cultural factors prevalence make the containment of resistance more challenging [27].
3. COMMON MECHANISM TO DEVELOP RESISTANCE IN MICROORGANISM
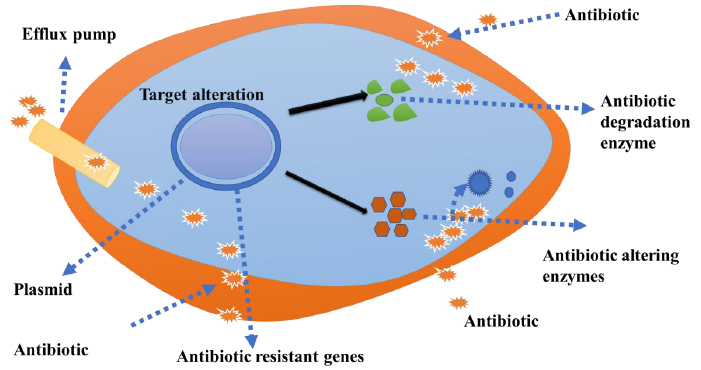
The key mechanisms of antimicrobial resistance include enzymatic degradation of antimicrobial produced by bacterial cells, a mutation in the antimicrobial targeting agents and prohibition of antibiotic entry inside the bacterial cells via membrane (Fig. 1). The spread of antibiotic resistance occurs through genetic material (plasmid or the bacterial chromosome) [28, 29].
4. ANTIBIOTIC RESISTANCE RATE IN MICROORGANISM
World Health Organization, European Centre for Disease Control and World Health Assembly highlighted the antimicrobial resistance as a major public health issue and will be a big challenge to grip for health care workers [30]. An establishment of multi-resistant strains arises due to the production of a broad spectrum of β-lactamases using various bacterial spp. after continuous consumption and became very common [31]. Antimicrobial resistance is an important public health concern at the global level. However, in India, recent hospital and community based reports showed an incensement of microbial resistance [32]. The reports showed varied resistance in different antibiotic resistance rates of various organisms along with various parts of India, as shown in Table 1.
5. CHALLENGES FOR ANTIMICROBIAL RESISTANCE
AMR in the world and emergence of newer Multi-Drug Resistant (MDR) and Extreme Drug Resistance (XDR) strains pose newer diagnostic and therapeutic challenges [33]. However, developed and developing countries are still striving to combat deep-rooted diseases such as tuberculosis, malaria and cholera pathogens, which are becoming with more drug resistant strains [34]. Several factors such as poverty, illiteracy, overcrowding and malnutrition further contribute to such kind of situation [35]. Lower healthcare professional to patient ratios is also involved for such kind of concern.
6. CASE STUDIES OF RESISTANCE DEVELOPMENT AND AFFECTING FACTORS
1. A study published by the Indian Council of Medical Research (ICMR) has found antibiotic resistant organisms of the digestive tracts in about 66% of the Indian population. The study was performed on 207 individuals with an analysis of stool samples, who had not taken any antibiotic from at least last month. Individuals selected were not suffering from any chronic illness. Isolates taken from 139 out of these 207 individuals were found to be resistant to one or more types of antibiotic classes. The maximum resistance rate was seen for cephalosporin (60%) and fluoroquinolones (41.5%) [36].
| Location | Isolates | Organism | Resistance rate (%) |
|---|---|---|---|
| Sikkim (2011) | 291 clinical Ssecimens, 196 carrier screening nasal samples | Methicillin-Resistant Staphylococcus aureus (MRSA) | 38.14 in clinical specimens, 20.92 in nasal samples |
| Tertiary trauma Center of AIIMS, New Delhi (2011) |
3,984 clinical specimens |
Gram-negative Pseudomonas, Acinetobactor, Klebsiella, E.coli, Enterobacter sp., Gram-positive S. aureus, Coagulase negative staphylococci |
Overall resistance of gram-negative organisms was 50 against carbapenems, 66 aminoglycosides, 76 Fluoroquinolones, 88 third generation cephalosporins |
| CMC Vellore, various centers across India (2010) | 176 clinical specimens |
P. aeruginosa | Among the 61 P. aeruginosa isolates, resistance to carbapenem was 42.6. |
| Puducherry (2010) | 31 clinical samples | K. pneumoniae | 93.55 multiple drug resistant and ESBL producer |
| Mangalore (2010) | 83 Community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) clinical isolates | CA-MRS strains | 92.8 were resistant to penicillin, 31.32 to erythromycin |
| Lok Nayak Hospital, New Delhi (2010) | 83 isolates from OPD cases of pyoderma |
CA-MRSA | 9.6 |
| Mangalore (2010) | 180 clinical samples | Enterococcal strains | 16.67 to 42.86 to aminoglycosides |
| Nagpur (2009) | 1300 nasopharyngeal swabs from school children | MRSA | 4.16 |
| Puducherry (2008) | 261 clinical isolates | 72.34 of Staphylococcus aureus resistant to oxacillin | |
| Lucknow (2007) | 2995 blood samples |
Klebsiella spp., Staphylococcus isolates | Extended-spectrum beta-lactamases (ESBL) producing Klebsiella spp. were 98.28 resistant to ampicillin, ticarcillin and piperacillin. Monobactam and cephalosporin resistance was also higher (>60) |
| Kolkata (2007) | 284 clinical isolates | Metallo-beta-lactamase (MBL) producing bacteria | 43.3 were resistant to at least seven antibiotics (ampicillin, amoxicillin, cephalexin, ciprofloxacin, cotrimaxazole, erythromycin,gentamycin) |
| MVIDH, Delhi (2007) | 9858 stool samples | V. cholera | 96 to furazolidone, cotrimoxazole and nalidixic acid |
2. A group of All India Institute of Medical Science (AIIMS), New Delhi, India researcher analyzed the water samples from seven different places in Delhi NCR regions along with the river, including its entry and exit points in the city, 35 bore wells and water percolating through waste. They found the concentration of dissolved drugs/antibiotics in the river water increased at an exit point rather than an entry point into the city. This case story concludes that either stopping or recommended use of the drugs/antibiotics by humans, animals and proper disposal method needs to be recommended to save the environment and welfare [37].
7. ANTIBIOTIC DOSE RECOMMENDED
Resistances against various classes of antibiotics were detected among various parts of the world [38]. However, the main reasons for this situation are either antibiotic doses not used under prescription or with proper treatment duration [18, 38, 39]. Increasing antimicrobial resistance is now a worldwide problem, compounded by the lack of development of new antimicrobial medicines [40]. Penicillin medication is used to treat several kinds of bacterial infections. It is also used for the treatment of a number of infections like pneumonia, bronchitis, gonorrhea and infections of the ears, nose, throat, urinary tract and skin [41]. Such type of treatments led to develop a resistance and side-effects against the penicillin class of drugs, when un-appropriate doses are given with no medical healthcare supervision/guidance [42]. The antibiotic should be prescribed only in case of bacterial infections, when the symptoms are severe, high risk of complications, and infection is not resolving [43]. Furthermore, in the case of severe infections, combinations therapy of antibiotics should be followed to achieve the synergistic effect of antibiotics at a lower dose under medical supervision.
8. GUIDELINES TO FOLLOW ANTIBIOTICS PRESCRIPTION
To prevent the development of antibiotic resistance, it is important to use antibiotics in the right way, to use the right drug with the right dose, at the right time for the right duration as per disease condition after diagnosis [44]. Prescribe the drug only where necessary, and consider benefits versus risks according to patient. The dosage should be prescribed carefully and be followed by manufacturer’s instructions [45]. The manufacture recommended doses are determined using clinical trial studies and assume one dose fits all [46]. The newer drugs are launched and necessary to review the patient to assess for effect, side-effects (Adverse Event; AE and Serious Adverse Events; SAE) and the need to continue [47].
9. PRECAUTIONS DURING ANTIBIOTICS UTILIZATION
1. Antibiotics are life-saving drugs, should be used safely and effectively [48]. During the entire course of any prescribed antibiotic, complete the course so that it can be fully effective and does not induce resistance [49]. It is required that necessary precautions should be taken by health care professionals to minimize the unnecessary prescribing and overprescribing of antibiotics [48, 50]. Many antibiotics such as amoxicillin, ampicillin, cephalosporin, etc. cause mild side effects such as diarrhea and abdominal pain, and need to be prescribed with appropriate stomach care supplement or probiotics [51]. It is recommended that, the prescribed treatment should be continuous, the side-effects of medicine may occur and subside gradually after a few days [52]. Try to practice good hygiene and use appropriate infection control procedures. Additional precautions should be taken for patients or suspected to be infected or colonized with highly infectious pathogens [53]. The usage of combination therapy would provide prevention against drug-resistant bacterial strains [54].
10. GOVERNMENT INITIATIVE POLICIES TO CONTROL ANTIBIOTICS RESISTANCE
(1) To prevent the misuse of important antibiotics, the Central Drugs Standard Control Organization (CDSCO), Govt of India has implemented Schedule H1 in India [55].
(2) Implementation of the National Health Policy, 2017 to public health and creating the awareness [56].
(3) Hospital infection control and prevention program [57].
(4) The regulation of antimicrobial’s sale [58].
(5) Training program to control antibiotic usage [59].
(6) An establishment of the National Centre for Disease Control (NCDC) [60].
(7) Antimicrobial stewardship (AMS) to prevent the emergence of antimicrobial resistance and decrease preventable healthcare-associated infections [61].
11. POSSIBLE STRATEGIES TO COMBAT THE PROBLEM ASSOCIATED WITH ANTIBIOTICS RESISTANCE
Followings strategies can be followed
(1) Understanding the emergence and spread of antibiotics resistance and key factors influencing it [62-65].
(2). Establishing a nationwide well-coordinated antibiotics program with well-defined and interlinked responsibilities and functions of different arms of the program [66-70].
(3) Rationalizing the usage of available antibiotics [38, 71].
(4) Reducing the antibiotic selection pressures by appropriate control measures [72].
(5) Promotion of discovery of newer and effective antibiotics based on current knowledge of resistance mechanisms [73-75].
(6) Rapid and accurate diagnosis of infections and infectious diseases [76, 77].
(7) Prescribe multiple antibiotics to achieve a synergistic effect at a lower dose.
CONCLUSION AND FUTURE PROSPECTS
AMR has increased the burden worldwide in pathogenic microbes, leading to the spread of microbial resistance across the globe. However, appropriate usage of respective antibiotics is the only way to prolonging life. A novel class of antibiotics needs to be discovered and introduced for better safety against severe microbial infection against drug-resistant species. Furthermore, novel molecular tools and diagnostics methods need to be explored to inhibit and develop the resistance in microbes. Additionally, the interaction of antibiotics with others should be studied in the laboratory to investigate the synergistic and antagonistic effects of antibiotics.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors greatly acknowledge Amity University, Noida, Uttar Pradesh, India, for providing the infrastructural support and opportunity to write this review.


