All published articles of this journal are available on ScienceDirect.
Amount of Milk Neutrophil Percentage and Associated CD Molecular Changes on the Compositional and Technological Properties of Milk
Abstract
Background:
Changes occurring in the activity and expression of neutrophils and their transmigration through the blood mammary barrier owing to a mammary infection affecting milk quality and outcome of mastitis.
Objective:
To understand the role played by various neutrophil molecules on mastitis and milk quality.
Methods:
18 Karan Fries cows of similar parity, milk yield and lactation stage were selected and screened for mastitis based upon milk Somatic Cell Counts (SCC) as well as California mastitis test and divided into 3 groups of 6 each i.e., healthy, sub-clinical (SCM), Clinical Mastitis (CM). Milk samples were analyzed for milk composition and technological properties. Milk neutrophils were isolated and their percentage, Phagocytic Activity (PA), viability were estimated. Activities of neutrophil enzymes i.e. Elastase 2, Collagenase and Cathepsin G were analyzed using ELISA. Relative mRNA expression of cell surface molecules like selectin (CD-62L), integrin (CD-11b), chemokine receptors (CXCR1 and CXCR2), CD-44 and chemotactic factor (IL-8) in milk neutrophil were also studied.
Results:
In the present study, neutrophil percentage (%) was significantly (p ˂0.05) higher in SCM and CM milk samples compared to healthy milk samples, whereas neutrophil PA, viability were significantly (p ˂0.05) lower in both samples contrast to healthy samples. Activities of Elastase 2 and Collagenase were significantly (p ˂0.05) more in SCM milk. There was a significant (p ˂0.05) difference in protein, pH, and lactose between healthy, SCM and SCM and CM milk. Significantly (p ˂0.05) high Electrical Conductivity (EC) was observed in CM milk than SCM and CM milk. No significant changes in milk fat, Solid Not Fat (SNF) and density were found among any of the groups. The relative mRNA expression of CXCR1, CXCR2 and IL-8 were significantly (p ˂0.05) high in milk neutrophils with the progression of SCM and CM, whereas significantly higher expression of CD11b CD-11b was found only in CM cows but there was no change in the expression of CD62L CD-62L in any of the groups. Expression of CD-44 molecule increased significantly in SCM cows, whereas it decreased significantly in clinically infected mastitis cows.
Conclusion:
This study highlights the changes occurring in the activity of milk neutrophils in healthy, subclinical and clinical mastitis crossbred cows.
1. INTRODUCTION
Mastitis is an infection of the udder, which has a high prevalence in dairy cows throughout the world. Mastitis occurs when a pathogen breaches the teat canal and invades the mammary gland. During the onset of mastitis, various leucocytes, particularly first line of cellular defense, i.e., neutrophils, get activated, are attracted and try to kill the invading pathogen. As the blood neutrophils transmigrate through the blood mammary barrier towards the affected mammary tissue, they not only breach the cellular architecture of the milk gland but also cause a decrease in milk lactose and variation in the milk proteins along with an increase in pH. These changes occurring in the milk quality decrease the shelf life of milk.
With the increasing demand for safe and nutritious food supply worldwide, research related to milk safety has taken a new urgency. More countries are working for a better understanding and effective management of mastitis. This research manuscript discusses one of the most important milk defense cells i.e. the neutrophil (first line of defense) and its role in influencing the removal of infection from the mammary gland and its subsequent effect on some of the milk quality parameters. Important molecules like selectin (CD-62L), integrin (CD-11b), CD-44, chemokine receptors (CXCR1 and CXCR2) along with IL-8 expressed on the surface of neutrophil have been highlighted.
2. MATERIALS AND METHODS
2.1. Selection of Animals
A total of eighteen crossbred Karan Fries (KF) cows maintained at Livestock Research Centre of National Dairy Research Institute, Karnal, Haryana, India were taken for the study. The average milk production in KF cow was around 3700 kg with 3.8-4.0% fat. All the cows were of similar parity, same average milk yield and in their early lactation (30-70 days). Milk was collected hygienically after cleaning the udder, and Somatic Cell Counts (SCC) were measured by somatic cell counter (Lactoscan). The cows were then divided into 3 groups viz.; healthy (n=6), subclinical (n=6) and clinical mastitis (n=6) according to their SCC and California mastitis test in them [1]. The cows were managed as per the practices followed in the institute’s herd and were offered ad lib green fodder and a calculated amount of concentrate mixture. Fresh tap water was available ad lib at all times of the day.
2.2. Milk Sampling
Milk samples were processed for neutrophil % and type of neutrophils i.e., immature neutrophils having band nuclei and mature neutrophils having segmented nuclei and also analyzed for the presence of probable mastitis causing organisms by pour plating method. The samples were subjected for the presence of S. aureus, E. coli, S. agalactiae, and total bacterial load. Briefly, the samples were serially diluted up to 10-7 dilutions. Nutrient agar was used for total bacterial load, Baird Parker agar used for S. aureus, Eosin Methylene Blue (EMB) agar for E. coli, and blood agar for S. agalactiae, followed by incubation at 37 °C for 24-48 h until the appearance of colonies. Samples which were positive only for S. aureus were selected for further processing.
2.3. Analysis
2.3.1. Estimation of Milk Composition and Somatic Cell Count (SCC)
Lactoscan milk analyzer was used for the estimation of milk composition of all the cows. SCC of milk samples was measured by a somatic cell counter (Lactoscan) and also cross-checked by making a milk smear and observing the milk smear glass slide under a microscope at 40× SCC of each original milk sample was determined in duplicate within 6 h post collection. The milk was heated to 40°C in a water bath held for 15 min at that temperature before being cooled to 20°C with careful stirring 0.01 ml of milk was spread on a 1 cm2 (0.5 cm × 2 cm) area of a degreased microscopic slide and was dried in a horizontal position. After drying overnight, duplicate smears were fixed with 96% ethyl alcohol (3 min), air dried, defatted with xylol (8 min), and rinsed smoothly with 60% ethyl alcohol and again air dried, followed by staining with pure May-Grunwald (2 min) and Giemsa solution (20 sec), air dried, and dehydrated in an increasing series of alcohols and xylols.
2.3.2. Phagocytic Activity of Milk Neutrophils
The in vitro Phagocytic Activity (PA) of milk neutrophils was estimated by Nitro Blue Tetrazolium (NBT) assay [2]. For this, neutrophils were isolated through density gradient centrifugation using Histopaque 1119 and Histopaque 1077 [3] and cells were collected at the interface of the Histopaque 1119 and Histopaque 1077 layers. These cells were then washed 3 times in PBS (300 × g, 10 min, 4 °C) and suspended in Roswell Park Memorial Institute (RPMI) media for further analysis. The cell suspension (neutrophils) was adjusted to 5 × 106 live cells/milliliter by the culture media (RPMI 1640). About 100 μl of the diluted cell suspension was placed per well in triplicates in a 96-well flat-bottomed tissue culture plate. The cells were allowed to proliferate with Zymosan (650 μg/ml) and NBT (250 μg/ml) concentrations that had been determined previously to provide maximal stimulation of bovine phagocytes [4]. In all the cases, the final culture volume was 200 μl. The blank wells consisted of 200 μl of culture media along with the same concentrations of NBT and zymosan. All cultures were allowed to incubate at 37 °C in a humidified CO2 incubator (95% air and 5% CO2) for 2 h. After that, OD was taken at 540 nm in a multi well-scanning spectrophotometer (Microscan MS-5608A).
2.3.3. Cell Viability Assay
The principle of this method is that dye can enter into the dead cells as their plasma membrane is disrupted and thus, the dead cells appear blue whereas, the dye cannot enter into the live cells because of their intact plasma membrane and the live cells appear colorless.
2.3.4. Neutrophil Enzyme Activity
The activities of three neutrophil enzymes viz., Elastase 2, Collagenase and Cathepsin G were measured by using various ELISA kits (WEKA MED and Wuhan Eiaab Science Co., Ltd., China) in milk neutrophils collected from healthy, subclinical and clinical mastitis cows. Neutrophils were isolated as discussed earlier in section 2.3.2. For the preparation of neutrophil lysate, the isolated neutrophils were dissolved in 1 mL PBS and glass beads were added to the neutrophil suspension. It was then shaken for 25 seconds using a Bead Beater (Unigenetics Instrument Pvt. Ltd., India) and immediately placed on ice for 1 minute, shaken again for 25 seconds followed by centrifugation at 1000 x g for about 10 minutes. The supernatant was taken in 2 mL Eppendorf tubes and stored at -20 ºC until further analysis. CV percentage was calculated from the calculated concentrations. Inter-assay % CV was found to be 4.36, 3.48, 6.03 and intra-assay % CV was found to be 2.17, 1.31 and 2.55 for the enzymes Elastase, Collagenase and Cathepsin G respectively.
2.3.5. Relative mRNA Expression of Neutrophil Genes
Total RNA extraction from isolated milk neutrophils was performed using TRIzols Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The integrity of the RNA was checked by agarose gel electrophoresis (2.5% agarose), and the quantity and quality of RNA were examined by nanodrop. Complementary DNA (cDNA) was prepared from 1 μg of RNA using the Novagen first strand cDNA synthesis kit (La Jolla, CA, USA) according to the manufacturer’s protocol. Synthesized cDNA was kept at -20 °C (or -70 °C for long-term use) till use.
Real-time quantitative reverse transcription PCR (qRT-PCR) was performed by Roche’s Lightcycler 480 instrument as per the methods of Pfaffl (2001) [5] with some modifications. Details of primers for specific bovine CXCR1, CXCR2, IL-8, CD-62L, CD-11b and CD-44 genes are shown in Table 1 (Sigma Chemicals Co., St. Louis, Missouri, USA).
For normalization of qPCR data, GAPDH was used as a reference gene. Reaction mixture for qRT-PCR was prepared as follows: 1 μL template; 5 μL (2×) SYBR green mixes, 0.5 μL each of reverse and forward primer, and 3 μL nuclease-free PCR grade water. The reaction was continued for 45 cycles at 95 °C for 15 s, annealing at 59 °C for 20 s, and performed the denaturation kinetics to assess the reaction product. All analysis was done using one-way ANOVA considering group as a factor by the SYSTAT software package. The relative expression ratio of the target genes was tested and analyzed for significance by the Relative Expression Software Tool REST version 2009 V2.0.13.
2.4. Statistical Analysis
Statistical analysis was performed as per the standard procedure. Statistical analyses were performed using the SPSS 17.0 statistical software package. The means were compared using Analysis of Variance and presented as mean ± standard error. The minimum significant range of confidence was evaluated at 0.05 level.
3. RESULTS AND DISCUSSION
The results of milk SCC and composition of healthy, SCM and clinically infected mastitis cows are presented in Table 2.
Somatic cell counting was done as it is widely used as a standard to ascertain the mammary health and milk quality [6] and is presented as cells per ml. Throughout the world, different countries have put a particular value of SCC to distinguish between healthy and mastitis milk [7]. In Indian conditions, values higher than 105 somatic cells/ml indicate a disturbance in mammary health [8]. In our study, there was a significant (p ˂0.05) difference in the milk SCC across healthy, SCM and CM cows and it was found to be highest in CM cows because of more intensity of inflammation. Significant increment of milk SCC in mastitis designates a protective mechanism to kill the invading pathogens in the mammary gland [9]. The number of somatic cells observed in healthy crossbred animals was found to be higher than indigenous cattle and lower to exotic cattle due to the difference in their milk production levels [10, 11]. Differential values in KF cows compared to other may also be due to variation in the shape of udder, teat and attachment of udder [12].
Coming to milk quality between healthy and CM milk, there was a significant (p ˂0.05) difference in protein, lactose, pH but no changes were observed between healthy and SCM milk and between SCM and CM milk. No significant changes in milk fat, SNF and density were found among any of the groups. A significant (p ˂0.05) change in the milk electrical conductivity was observed among healthy, SCM and clinically infected mastitis cows. An increase in protein percentage of the infected milk may be due to more secretion of blood-borne proteins into milk as a result of disruption of blood mammary integrity and opening of the tight junctions by the bacterial toxin [13] and it is associated with an increase in level of whey proteins and decrease in different caseins in mastitis milk which leads to poor curdling and lowered cheese yield [14]. A decline in lactose content in mastitis milk may be due to the damage to the mammary epithelial cell and leakage out of milk through paracellular pathways [15]. Udder inflammation leads to decrease in secretory activities of epithelial cells and increase in permeability. Hence, there is a possibility of transfer of higher amount of citrates, bicarbonates from blood to milk which might be a possible cause to the higher pH in milk of mastitis origin [16]. In addition to this, there is a chance of an increase in the concentration of sodium and chloride above normal level in mastitis milk compared to healthy milk and thus increases the electrical conductivity [17].
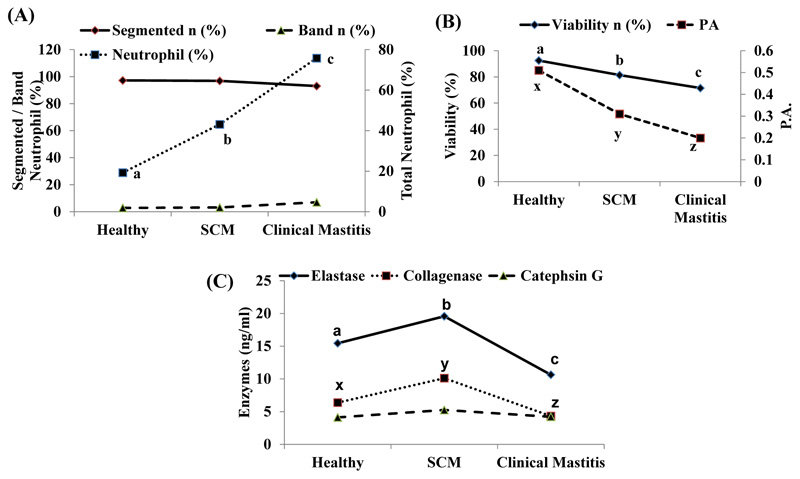
In the present study, neutrophilic activity was studied in detail because neutrophils are the first line of cellular defense. The activity and influx of neutrophils into the mammary gland influences the rate and duration of an infection. Owing to infection, neutrophils are the first cells to migrate from the blood into an inflamed area after initiation of inflammation. In our study, there was significantly high (p ˂0.05) percentage of milk neutrophil in SCM and CM cows compared to healthy cows. Segmented and band neutrophils percentage was also found to be higher in mastitis group in comparison to healthy and SCM cows (Fig. 1A).
It occurs due to migration of more immature neutrophils from the bone marrow to the mammary gland to fight infection [1]. However, due to immaturity in their development, they cannot exhibit their full potential. The main function of neutrophil is phagocytosis and intracellular killing. Compromised phagocytic ability of milk neutrophil predisposes more chances of mastitis [2]. Moreover, in our study, we observed significantly lower viability, as well as phagocytic ability in SCM and CM cows compared to healthy cows and lowest values, were in clinical mastitis group (Fig. 1B). Scanning Electron Microscopy (SEM) studies carried out on neutrophils in out aboratory have revealed that there was adistinct change in the morphology of milk neutrophil comparedto blood neutrophil [6]. Milk neutrophils displayed fewerpseudopodia formation/less surface roughness than blood neutrophils. So they have poor phagocytic activities as pseudopodia help in the formation of phagolysosome and thus phagocytosis. Simultaneously, they exhibit non-pathogenic phagocytosis by engulfing casein and fat globules. Transmission electron Microscopy Study (TEM) indicated that the decrease in the number of cytoplasmic granules in milk neutrophils (as they wrongly participate in the phagocytosis of casein and fat globules) further aggravates their poor Phagocytic Activities (PA) [18]. In addition to this, less glycogen reserves curtail their life span. After that, they succumb to program cell death i.e. apoptosis which on a positive note helps to create less havoc to mammary gland architecture.
| Genes | Sequence (5′→3′) | Primer Sequence Length | Acc. No. | Size (bp) | Basic Temp ( 0C) |
|---|---|---|---|---|---|
| CXCR1 | For GTCCCCGTGAGATAAGCAC Rev CAGGTTCAGCAGGTAGACA |
20 | EF597244.2 | 163 | 59 |
| CXCR2 | For CAACACTGACCTGCCCTCTA Rev CAGGTTCAGCAGGTAGACA |
20 | DQ328664.1 | 197 | 58 |
| IL-8 | For TGCTCTCTGCAGCTCTGTGT Rev CAGACCTCGTTTCCATTGGT |
20 | EU276073.1 | 190 | 59 |
| CD-11b | For AAACTGGCAGAAAGCAACA Rev CCAGGAAGACTCTGGAGGA |
20 | NM_175781.1 | 183 | 58 |
| CD-62L | For CCGATTGCTGGACTTACCAT Rev CCAAGTCCACACCCCTTCTA |
20 | NM_174182.1 | 194 | 59 |
| CD-44 | For CCAGAAGGAACAGTGGTTTGGC Rev ACTGTCCTCTGGGCTTGGTGTT |
22 | NM_000610 | 140 | 59 |
| GAPDH | For GGGTCATCATCTCTGCACCT Rev GGTCATAAGTCCCTCCACGA |
20 | NM_001034034 | 176 | 59 |
| Healthy | Subclinical Mastitis | Clinical Mastitis | |
|---|---|---|---|
| SCC (×103) | 166.60 ± 31.57a | 460.16 ± 32.74b | 750 ± 31.62c |
| Fat (%) | 4.14 ± 0.06 | 4.33 ± 0.09 | 4.25 ± 0.06 |
| Protein (%) | 3.31 ± 0.09a | 3.55 ± 0.06ab | 3.61 ± 0.03b |
| Lactose (%) | 4.85 ± 0.13a | 4.55 ± 0.07ab | 4.30 ± 0.04b |
| SNF (%) | 9.76 ± 0.25 | 9.47 ± 0.21 | 9.22 ± 0.14 |
| pH | 6.62 ± 0.02a | 6.76 ± 0.02a | 6.92 ± 0.07b |
| EC | 5.44 ± 0.05a | 6.48 ± 0.06b | 7.43 ± 0.06c |
| Density | 32.18 ± 0.88 | 31.14 ± 0.72 | 30.29 ± 0.49 |
Besides phagocytosis, degranulation is also one of the major neutrophil killing mechanisms. However, degranulation also causes the modification of milk proteins resulting in poor milk quality [19]. Specific neutrophil enzymes like Elastase and Cathepsin G are found in the azurophilic granules, whereas collagenases are present in the specific granules. Enzymes Elastase and Cathepsin G are serine proteases that help in the destruction of pathogens [20]. Enzyme activity of three neutrophilic enzymes viz., Elastase 2, Collagenase and Cathepsin G in healthy, SCM & CM cows has been presented in (Fig. 1C). Activities of Elastase 2 and Collagenase were significantly (p ˂0.05) high in SCM cows than healthy and CM cows. However, there was no significant change in Cathepsin G activity among the groups.
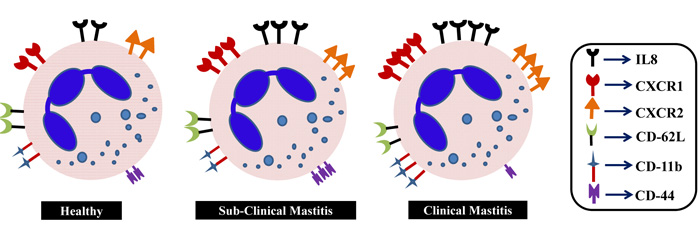
Being the infantrymen, the neutrophils have to migrate rapidly towards the inflammatory site so as to check the infiltration of intruders as early as possible. It provides sufficient time to the second line of defense to counter with its full potency. Contrary, it is also important that neutrophils have to clear off from the site so as to avoid damages to the mammary gland because of their secretary content during the killing process. To understand this, we framed the molecular study pertaining to the transmigration of neutrophils. For that, we studied various molecules like chemokine receptors (CXCR1 and CXCR2), IL-8 and CD molecules (CD-11b, CD-62L and CD-44) expressed on milk neutrophils and their changes in expression under healthy, SCM and CM condition. The schematic presentation is depicted in Fig. (2).
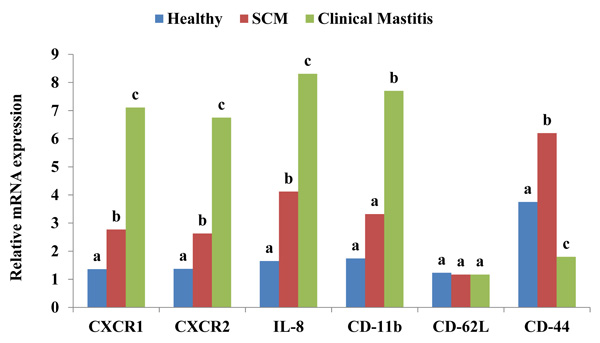
After receiving a chemotactic signal from the invading organism, neutrophils respond to inflammation through 3 classes of surface proteins i.e. chemokines, selectins and integrins. CXC chemokine receptors present on neutrophils are integral membrane proteins that specifically bind and respond to cytokines of the CXC chemokine family. CXCR1 selectively binds only to IL-8 with high affinity, while CXCR2 binds to IL-8 and other chemokines such as growth related oncogene-α and neutrophil activating peptide-2 [21]. CXCR2 is also expressed on neutrophils and is associated with the efficiency of neutrophil migratory viability. Activation of CXCR2 by its cognate ligands induces intracellular signals associated with chemotactic migration and recruitment and infiltration of neutrophils from the blood stream during inflammation [22]. IL-8 is known as neutrophil chemotactic factor which is released in response to cell stressors such as reactive oxygen species, bacterial fragments and pro-inflammatory cytokines [23]. IL-8 recruits neutrophils to the site of infection and allows the host to be more effective in fighting an infection. When IL-8 bounds to neutrophil, it leads to conformational changes in the neutrophils which allow its adherence to endothelial cells. This causes exocytosis of soluble storage proteins from secretory vesicles and granules causing increased expression of adhesion molecules such as CD-11b, c and CD-18 that are essential for adhesion to endothelial cell [24]. Expressions of CXCR1, CXCR2 and IL-8 were significantly (p ˂0.05) high in the milk neutrophils with progression of SCM and CM as presented in Fig. (3). It represents the chemotactic rapidity of neutrophil towards an inflammation. Verbeke et al. (2015) [25] reported an up-regulation of CXCR1 when the quarters of Holstein cows were inoculated with S. chromogenes. Another study suggested that when bovine mastitis occurred, the level of CXCL8 and its mRNA expression increased rapidly with a peak at 24 h [26]. CD-62L mainly mediates rolling action on endothelial layer whereas, CD-11b allows adherence of neutrophils to the endothelial cells. In our study, significant (p ˂0.05) increase in the expression of CD-11b was observed only in case of CM cows. No change was observed in the expression of CD-62L, in the milk neutrophils of healthy, SCM and CM cows (Fig. 3).
Higher expression of CD-11b is essential to recruit more number of neutrophils towards infection [27]. Lower expression of CD-62L may be due to shedding of these molecules after crossing the blood mammary barrier and compared to blood neutrophils milk neutrophils exhibit less expression [28]. The relative mRNA expression of CD-44 was significantly (p ˂0.05) high in SCM whereas, reduced expression was observed in CM cows (Fig. 3). The possible reason for higher expression in SCM cows might be to protect the mammary gland as macrophage does CD-44 assisted neutrophil removal from the site of infection [29]. Lower expression of CD-44 on the surface of neutrophil in CM cows can be correlated with their delayed removal as one of the causes of secondary inflammation and tissue injury during CM condition. Extensive research is required to explore and understand its complete physiology.
CONCLUSION
Concurrent to the negotiation of food security challenge concerns over food quality and safety measures have been a critical issue globally. As dairy sector has become an important segment of food industry, providing safe and nutritious milk, this aspect has become increasingly important. Quality and production of milk, a balanced food, are hampered because of mastitis which is the most common and prevalent disease in dairy cows causing huge economic losses. The real challenge to maintain the quality of milk in India is related to the fact that the major amount of milk is produced by cows with subclinical mastitis because the signs and symptoms of animals go unrecognized. In addition to this, even after subsiding of mastitis, the animals cannot get back to its full milk production potential due to excessive damage of the mammary gland epithelial cell. Thus, our study is proposed to target the milk cells, and particularly the neutrophils, with the aim to recognize rapidly milk from cows in healthy, subclinical and clinical mastitis conditions. This paper highlights the changes occurring in the number and activity of milk neutrophils in healthy, subclinical and clinical mastitis crossbred cows. Clinical mastitis cows had more number of immature neutrophils, whereas the enzymatic granule responsible for attacking the pathogens and their phagocytic activity was lower. Expression of neutrophil markers like CXCR1, CXCR2, IL-8 and CD-11b were also seen to be elevated in cows with naturally occurring mastitis. The modulation of the neutrophil functions as given above may be associated with the progress of mastitis from subclinical to clinical stage and help in understanding the infection dynamics of the mammary gland of a cow. These research findings need to be explored further and implemented in complete udder health management programs in large number of dairy animals. In future, these findings might be extended for the development of some novel and efficient diagnostic methods to prevent this economically impacting disease of mastitis affecting dairy cows worldwide.
AUTHORS’ CONTRIBUTION
Mohanned Naif Alhussien performed the experiments; Bibhudatta S. K. Panda and Sunil Kumar Mohapatra analyzed the data and wrote the manuscript whose final version was approved by Dr. A. K. Dang.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Livestock Research Centre of National Dairy Research Institute, Karnal, Haryana, India.
HUMAN AND ANIMAL RIGHTS
No humans were used in the study. The experiment on animals was in accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) rules, laid down by the Government of India.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank the Director of the Institute for providing all the facilities to carry out this research work.


