All published articles of this journal are available on ScienceDirect.
Quartz Crystal Microbalance Detection of Aflatoxin B1 by Self-Assembled Monolayer Technique
Abstract
Background:
New biosensor techniques allowing detection of low concentrated substances show a great variety nowadays. The construction of a system with modified quartz as a part of a Quartz Crystal Microbalance (QCM) techniques helps the detection and confirmation of low toxin concentrations in a sample.
Objective:
The study aims to allow the application of methods for preparation and modification of the gold surface of piezoelectric crystal for detection of aflatoxin B1(AFB1) in the concentration range 0.2 - 2.0 µg/L by QCM technique (quartz - crystal microbalance).
Methods:
The procedure for the preparation of quartz crystal sensors for experimental purposes was performed. The quartz surface was activated and covered with self-assembled monolayer to immobilize antibody (rabbit anti-aflatoxin B1) for the detection of antigen - antibody reaction.
Results:
The G” corresponds to viscous properties of the material, during applied deformation of the material in the presence of different concentrations, which revealed in the sensitivity of the used resonator.
Conclusion:
Detection of toxic pollutants may be achieved via QCM methods, ultrasound resonator and piezoelectric quartz techniques for measurement. These techniques allow detection of significantly low concentrations of toxic pollutants, in particular, AFB1, compared to analysis with direct and indirect ELISA immunoassays.
1. INTRODUCTION
Typical representatives of mycotoxins are aflatoxins. They are di-furanocoumaric derivatives produced by Aspergillus flavus, Aspergillus nomius, Aspergillus niger and Aspergillus parazit [1]. AFB1 is a potent hepatotoxicant and liver carcinogen. Since it acts through a genotoxic mechanism, a tolerable daily intake cannot be set and human exposure should be reduced to levels as low as achievable. Tolerable levels (in the range from micrograms to nanograms per kilogram) have been set in Europe in various susceptible plant-derived food commodities, as well as feed materials and complete feeds, based on the calculations of margins of exposure [2, 3]. The first reported case on aflatoxin M1 contamination was in ovine milk, after feeding contaminated feed with AFB1. This hepatic hydroxylated form of AFB1 was originally called “milk toxin” [4]. Several investigations report that four main aflatoxins occur: B1, B2, G1, G2, plus two more metabolic products М1 and М2, which are significant in the role of direct contaminants of food and fodder. Aflatoxins М1 and М2 were initially isolated from milk and animals [5]. Exposure by ingestion or inhalation of aflatoxins may lead to serious medical conditions that vary considerably depending on the animal species, dose, diet, age and gender [6, 7].
The highest risk for contamination with aflatoxins goes to crops (wheat, rye, barley, corn, etc.), nut fruits (peanuts, pistachios, and almonds), milk and milk products. The lowest risk for contamination with aflatoxins corresponds to dried fruits (figs, raisins, etc.) and spices. The acceptable standard for aflatoxin content in pistachios is 4 mg/kg whereas, in particular, the maximum acceptable quantity of aflatoxin B1 is 2 mg/kg [8].
The current investigation concerns finding appropriate methods for detection of affinity binding of antigen - antibody reaction and detection of influence of different concentrations of mycotoxins in milk and water samples. Using QCM analysis and technique for the formation of self-assembled monolayers onto piezocrystals with gold coverage, the following experiments were examined: detection of antibody - antigen reaction and detection of mycotoxins in pre - contaminated samples of milk, yogurt and water. Thus, the network analyzer can be used as a biosensor for the determination of toxic pollutants in industrial practice. The current work focuses on the development of an immunosensor for the detection of AFB1 based on antigen-antibody reaction.
2. MATERIALS AND METHODS
2.1. Materials
The network analyzer Agilent 4395 A with module QOM 401 (Q-Sense Open Module) was kindly provided by the laboratory SATIE, University of Cergy - Pontoise, France. QSX 301 crystal sensors with gold coverage, resonated from 5 - 15 MHz were acquired from Biolin, scientific company. Quartz solution was purchased from Helmanex. 1-ethyl-3-[3-(dimethylamino) propyl carbodiimide hydrochloride (EDC) and N-hydroxy sulfoximide (NHS) were purchased from Sigma Aldrich.
Rabbit anti-aflatoxin B1 and aflatoxin B1-BSA conjugate from Aspergillus flavus were obtained from Sigma Co., Ltd. Monoclonal anti-aflatoxin B1, clone AT-B1 produced in mouse, ascites fluid (Product No A9555) and antiaflatoxin B1-peroxidase antibody produced in rabbit (Product No A2681) were acquired from Sigma Co., Ltd. Streptavidin – peroxidase was purchased from ScyTecLaboratories. Ethanolamine and 3-mercaptopropionic acid were obtained from Sigma Aldrich. All the used solvents for the obtained measurements were analytical grade.
2.2. Procedure for Preparation of Quartz Crystal Sensors for Experimental Purposes
QSX 301 crystal sensors with gold coverage, resonated from 5 - 15 MHz, were used. The quartz surface was prepared according to the consecutive procedure, described below. The first step of the procedure was cleaning the quartz surface with “Pr Quartz solution” for 5 min. Then the quartz was rinsed several times with distilled water, followed by washing with isopropanol for 10 min at 50°C. The last step was quartz drying at 50°C. The quartz was placed in thermostat for calibration of the oscilloscope. The dry and tempered quartz was calibrated as a control one in the module cell connected to ultrasound analyzer.
2.3. Activation and Coverage with Self-Assembled Monolayers (SAM) onto Quartz Surface for Current Antibody Immobilization
2.3.1. Preparation of Piezocrystal QSX 301 for Activation of Gold Surface
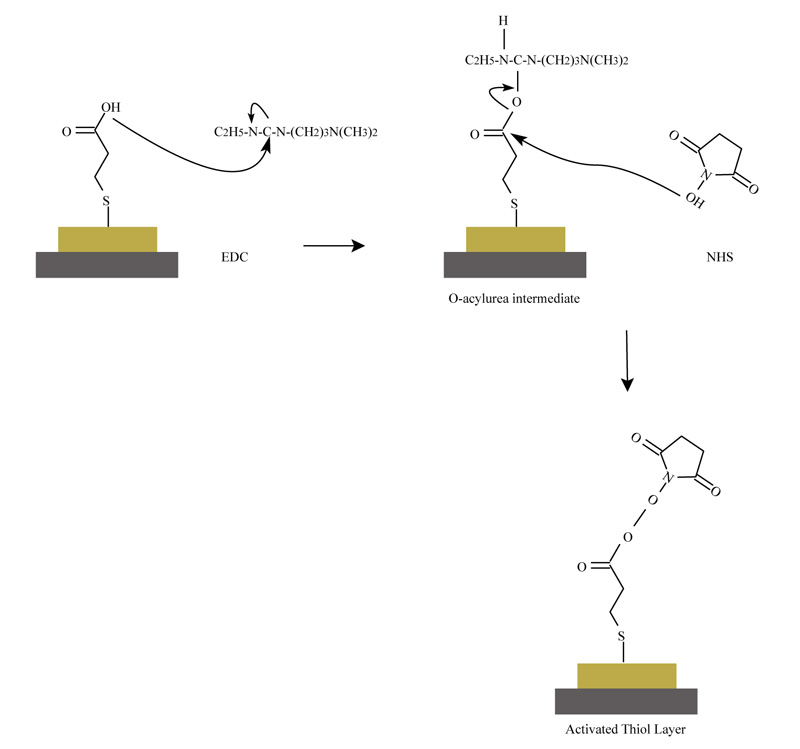
The consistent reactions during preparation and activation of quartz surface are presented in Fig. (1), according to the described and cited procedure:
The quartz was rinsed with a solution of 1.2M NaOH for 5 min and it was washed several times with distilled water. Further, the quartz was treated with 1.2M HCl for 5 min and it was washed with distilled water several times. The quartz was steeped in concentrated HCl for 1 min. The working protocol required washing and drying of the treated quartz again. The last step was to immerse the quartz in 10 mM 3 - mercaptopropionic acid for 24 h. After this procedure, the quartz was rinsed and sonicated in absolute ethanol for 10 min. The crystal was washed and dried to remove any unnecessary adsorbed monolayered structures. The described procedure was made before each experiment in order to activate the quartz surface [9].
2.3.2. Activation of Monolayers onto QSX 301 Piezocrystal Surface
The quartz was placed in a module cell. 100µl 1-Ethyl-3-[3-dimethylamino propyl] (EDC) carbodiimide hydrochloride) and 100 µl N-hydroxysulfosuccinimide (NHS), were pipetted sequentially. NHS was added, in order to replace the activated hydroxyl groups of EDC as a result of the formed oxygen - acyl - carbamide intermediate structures [10]. Self-Assembled Monolayers (SAM) were formed onto the crystal surface. The used reagents dissolved in distilled water with a concentration 100 mg/µl. The treated quartz was kept for 1 h. The detection of the sequential addition of the solutions of EDC and NHS onto quartz surface was done with ultrasound resonator, in owing to follow and approve the change of signal in every step (Fig. 1).
2.4. Immobilization of Antibody (Rabbit Anti-Aflatoxin B1) and Detection of Antigen - Antibody Reaction
The activated quartz surface was washed with distilled water and phosphate buffer (0.01M, pH 7.4). 10µl of antibody solution (rabbit anti-aflatoxin B1 dissolved in 0.01M phosphate buffer, pH 7.4, containing 15 mM NaN3, and diluted 1:1000) and 90µl phosphate buffer were pipetted. The change in the signal of piezoelectric in 1 hour time was observed by the quartz resonator. The quartz surface was washed with distilled
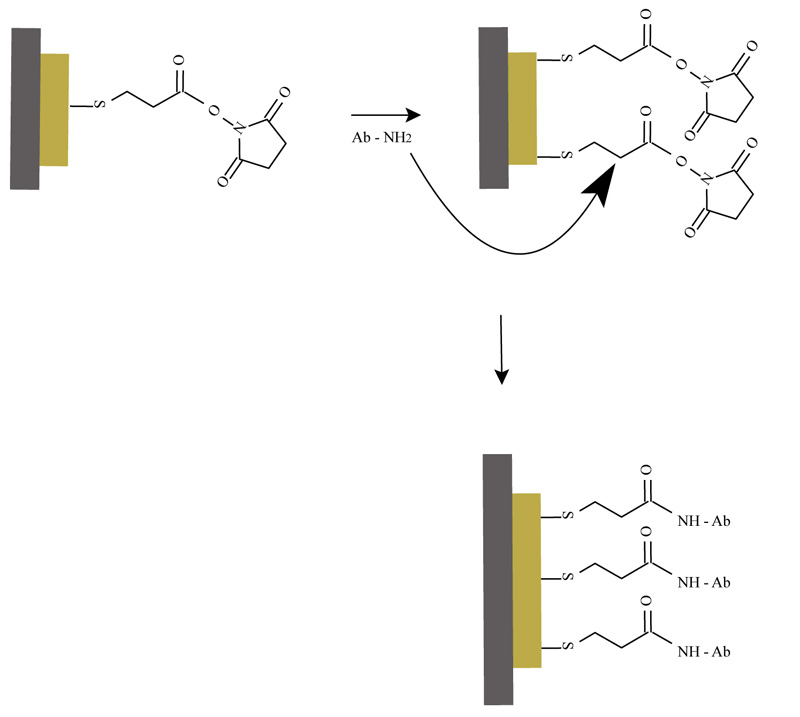
water and phosphate buffer. Two types of blocking agents were used: 100µl ethanolamine (10mM) and 100µl Bovine Serum Albumin (BSA). The blocking solution reacted in the module cell for 1 h and let the blocking of the additional groups onto the quartz surface. The piezoelectric response, as a result of the separated addition of the blocking agents, was followed by the resonator.
The process of immobilization of the rabbit anti - aflatoxin B1 is presented in Fig. (2).
Once the activated monolayer is prepared, several molecules of the antibody can be attached on the surface. The amine groups of the antibodies replace the NHS and that follows to a formation of covalent bonds. This method is useful for the detection of antigen in a sample. The signal from the antigen-antibody reaction is measured and transformed by the resonator (Fig. 2). The SAM technique for immobilization of protein molecules can be used for the preparation of different immunosensors, based on QCM detection techniques analysis.
2.5. Preparation of Milk Samples and Measurement of AFB1
1.1 g of skimmed milk powder was measured and diluted in 9.990 ml distilled water. The sample was stirred and heated to 90°C for 10 min. Just before the measurement in the module cell, the milk sample was cooled to 25°C [11] and contaminated with a relevant concentration of AFB1-BSA, till the final volume of 10 ml (final concentration of AFB1-BSA, respectively 0.2 µg/L and 2 µg/L). The measurements were done with piezocrystal, which resonated to 15 MHz. The sample was measured in the presence of antibody, immobilized onto the quartz surface without pre–blockage of free groups on the surface.
After activation of quartz surface and immobilization of antibody, the crystal was washed with distilled water and phosphate buffer. 10µL of pre–contaminated sample with a concentration of AFB1-BSA, respectively 0.2 µg/L and 2 µg/L, were pipetted on the quartz in the module cell. Further, it was added 90µl phosphate buffer. The measurements and piezoelectric responses of quartz were monitored in real-time.
3. RESULTS AND DISCUSSION
3.1. Observation of Piezoelectric Response of the Sensor Due to the Antigen - Antibody Reaction
After the blockage of non-bonded active groups of activated quartz surface and washing of the quartz in a certain time frame, the detection of antigen - antibody reaction was observed. Concerning the minimal range of concentrations of antigen, we started the experiment with 100 µg/ml stock solution of AFB1-BSA. The addition of 10 µl, 30 µl and 100 µl of stock solution of antigen in phosphate buffer with ratio 10:90, 30:70, was estimated by the quartz resonator. The molecular weight of AFB1 is around 300 g/mol. We detected AFB1, conjugated with BSA by QCM to follow the antigen-antibody reaction and make a comparison with ELISA immunoassay. The QCM detection of AFB1 with unknown concentrations is influenced by pre-determined concentrations of AFB1-BSA. When the AFB1 concentration in the sample is low, then a number of AFB1-BSA molecules may interact with the immobilized antibody on the quartz surface, which will result as a higher signal of the resonator’s response. The reaction antigen - antibody in the module cell of the resonator was monitored for 24 h (Fig. 3-7).
All sensor’s responses were measured in real-time and the results were graphically presented, applying a developed mathematical model for processing the data in the laboratory of SATIE, University of Cergy - Pontoise [12, 13].
According to the presented graphs, we can see that the essential difference in resonator’s responses between the antigen – antibody reaction occurs at concentrations of antigen 10 µg/ml (Figs. 3) and 100 µg/ml (Fig. 5) of AFB1-BSA.
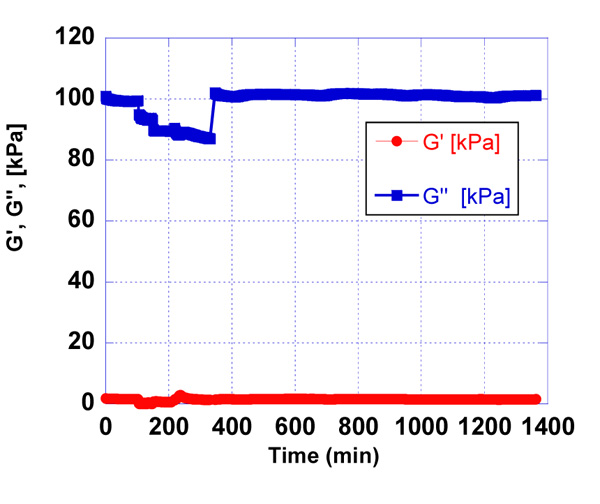
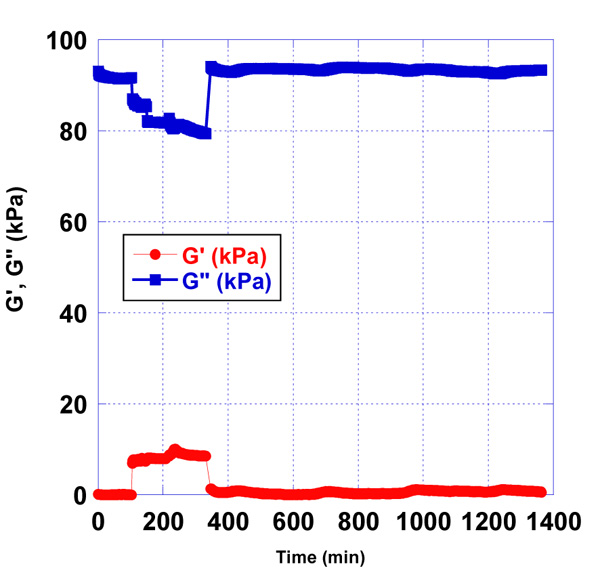
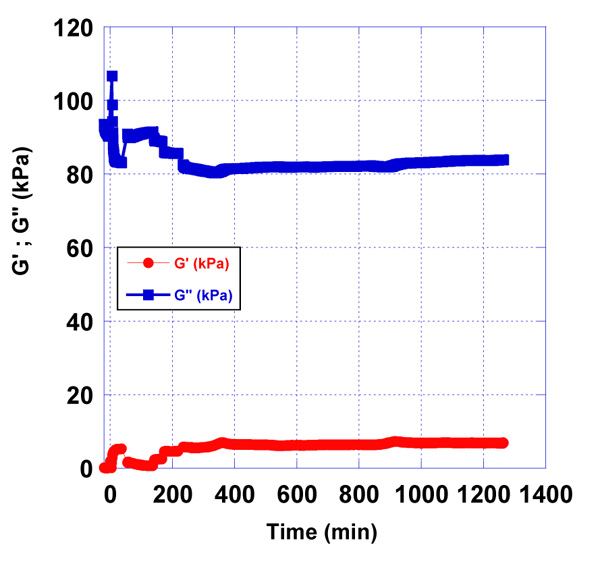
3.2. Detection and Measurement of Different Concentrations of AFB1-BSA Conjugate in Pre-contaminated Milk Samples
In addition to the detection of antigen – antibody reaction, followed by the piezoelectric response in real-time using oscilloscope Agilent 4395 A, we also observed changes in viscoelastic properties of matter, covering the activated quartz, by measuring different concentrations of AFB1-BSA in pre – contaminated milk samples. Most of the published data about concentrations in food sources show that the accepted limit range for AFB1 content in food samples is in the range from 0,02 µg/L to 0.3 µg/L.
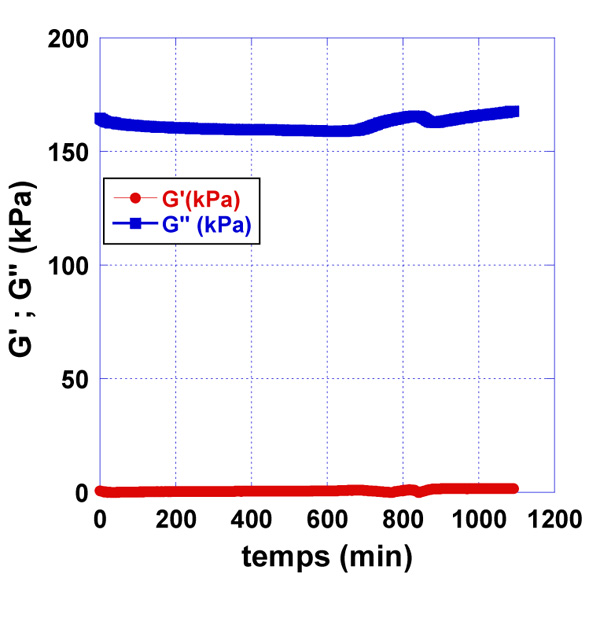
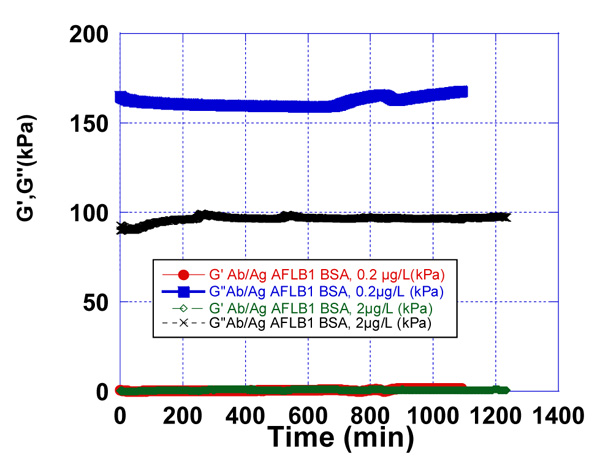
We observed the effect of the immobilized rabbit anti-aflatoxin antibody B1 to the quartz surface in the presence of pre-contamination with AFB1-BSA milk samples. The used concentrations of AFB1-BSA were 0.2 µg/L and 2 µg/L (Fig. 6-8).
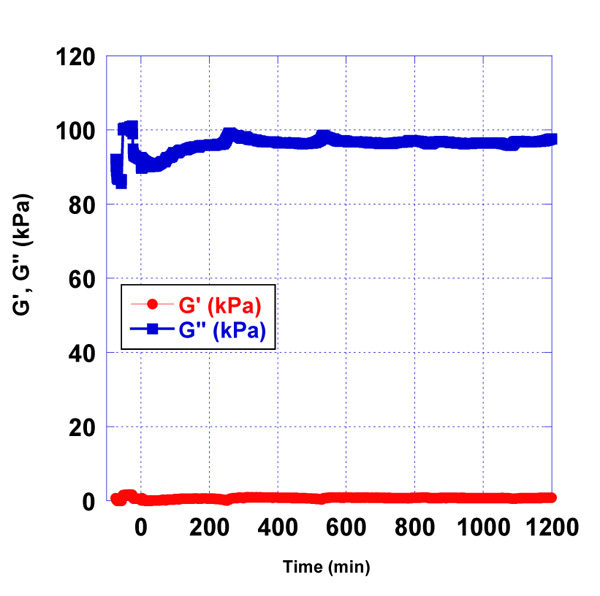
Sample of pure milk, without AFB1-BSA was prepared as a control sample and the resonator signal was measured. In addition, as a control sample was used the water samples, contaminated with 0.2 µg/L and 2 µg/L AFB1-BSA respectively. Control water samples were used, corresponding to the Newtonian fluids, in which viscosity is only dependent on temperature differences. Those samples have linear raise in stress with increasing shear rates and their viscosity remains constant. Water samples were used to follow the minimal changes in signals on the quartz surface in the presence of contaminated samples with AFB1-BSA. Milk samples were prepared from skimmed milk. Those samples are considered also like Newtonian fluids. Compared to water, skimmed milk has a plenty of protein, so we used the control water samples to see the differences of signals on the quartz resonator.
After processing the obtained data as a result of the experiments, differences in the accumulation module - G ” were observed, concerning the applied concentrations of AFB1-BSA (0.2 µg/L and 2 µg/L). This is a prerequisite for confirming the sensitivity of the used resonator.
3.3. Immunodetection (ELISA)
Indirect and direct ELISA immunoassays were used as comparative methods for the detection of AFB1.
The protocol for analysis included overnight incubations of different concentrations of AFB1. The mycotoxins’ solutions were prepared in binding buffer (50 mM Na2CO3, pH 9.6), with concentrations of AFB1 in the range of 3 g/L to 0.001 g/L. Concentrations lower than 1 mg/L did not show any mathematical relevance between the measured minimal concentrations of AFB1. 50 μl samples were pipetted in each well of the microtiter plate for ELISA. In the next morning, the plate was blocked with 3% BSA in PBS-T (0.05% Tween-20 in PBS).
We used antiaflatoxin B1-peroxidase antibody produced in rabbit for direct ELISA analysis, diluted in ratio 1:1000 in PBS-T. The serum incubation was processed for 2 hours at room temperature. The color reaction was developed with OPD (о-phenylendiamine dihydrochloride) in citrate buffer (pH 5.00). After 30 min the reaction was stopped with 10% solution of 2N H2SO4. The absorption was detected on ELISA reader at 450 nm. In the case of indirect ELISA, we used mouse monoclonal antibody against aflatoxin B1, AT-B1, ascite produced in mouse (dilution ration 1:20000). After overnight incubation of the antibody, we additionally incubated the plate with anti - mouse antibody, conjugate with biotin. We used streptavidin - peroxidase for maximal enhancement of the signal and reaction development. The color reaction was developed respectively to the procedure of direct ELISA (Fig. 9).
The sensitivity of direct and indirect ELISA for detection of AFB1 is very low, which can be seen from very low linearity of the signal for concentrations, lower than 1000mg/L.
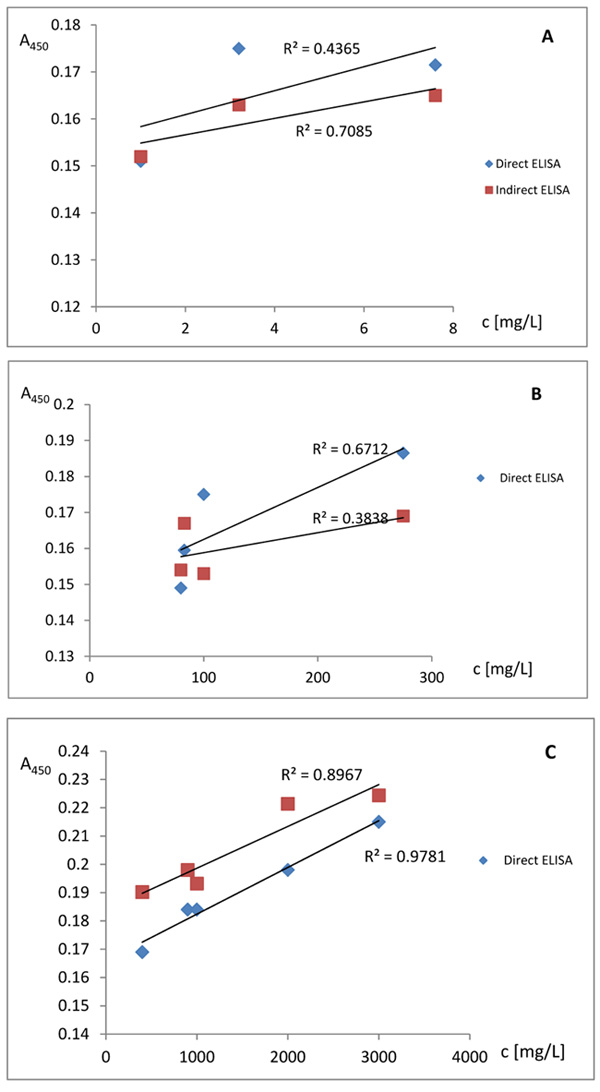
A-concentration of AFB1 in the range 1 - 7.6 mg/L;
B-concentration of AFB1 in the range 80 - 275 mg/L;
C-concentration of AFB1 in the range 400 - 3000 mg/L;
CONCLUSION
The experimental results showed that QCM biosensor measured concentrations of the mycotoxin in the range of 0,2-2,0 µg/L. The same range of sensitivity for the detection of AFB1-BSA with direct and indirect ELISA is not observed. ELISA method is the indirect method, corresponding to the conjugation of enzyme or fluorochrome. Below 1000 mg/L concentrations of AFB1, low linearity is observed. In comparison, QCM can be used for the construction of biosensor with direct detection of the analyte and for parallel analysis in a few minutes. Furthermore, QCM can be used for the detection of AFB1 in the range of 0,2 µg/L-2,0 µg/L. With ELISA method parallel analysis of several samples is not possible. QCM analysis by the ultrasound resonator with piezoelectric quartz can be used for the construction of new types of immunosensors for toxic pollutants detection. These measurement techniques allow the detection of significantly low concentrations of AFB1 which cannot be achieved with direct and indirect ELISA.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The present study was supported by project DN 07/21 FUND “Scientific Investigations”, Bulgaria.
Presentation of experimental results has been financially supported by the Operational Programme “Science and education for smart growth”; 2014-2020 of the European Union cofounded by the European Social Fund through the project BG05M2ОP001-2.009- 0015 “Support for the development of capacity of doctoral students and young researchers in the field of engineering, natural and mathematical sciences”.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


