All published articles of this journal are available on ScienceDirect.
Indirect Labeling of Antibodies as a Universal Approach to Increase Sensitivity of Lateral Flow Tests: A Case Study for Mycotoxins Detection
Abstract
Objective:
The study aimed at increasing the sensitivity of immunochromatographic tests for the control of toxic contaminants (on the examples of aflatoxin B1 and T-2 toxin) in agricultural products.
Methods:
For reliable immunochromatographic detection of low concentrations of analytes, a replacement of the (specific antibodies – gold nanoparticle) conjugate by a combination of native specific antibodies and anti-species antibodies conjugated with gold nanoparticles was proposed. Different variants of test systems based on the principle of indirect labeling were realized and compared.
Results:
Immunochromatographic assays with indirect labeling for aflatoxin B1 and T-2 toxin were implemented experimentally. A reduction in the detection limit by one to two orders of magnitude was demonstrated.
Conclusion:
The presented results confirm that indirect labeling of specific antibodies overcomes the limitations of the competitive immunochromatographic analysis and can be used to detect analytes of different chemical nature.
1. INTRODUCTION
1.1. State of the Art with the Development and Use of Lateral Flow Tests
Actually, food safety is an extremely important concern for modern society [1, 2]. New tools for rapid on-site detection of toxic contaminants in food and agricultural products are highly requested for technological control and health protection of consumers [3-6]. Among different proposed techniques, lateral flow tests seem to be perspective tools for wide primary screening control due to their rapidity, simplicity and low cost [7-9]. The lateral flow tests realize a principle of Immunochromatographic Assay (ICA) with preliminary application of all the reagents to a multi membrane composite (a test strip)
and initiation of the assay by contact of the test strip with a liquid sample to be tested with the following movement of the reactants along the test strip, the formation of labeled immune complexes and their detection as colored zones of the test strip [10]. All these processes are implemented in 10-20 minutes, and the assay results (presence or absence of the coloration) may be visually assessed. The conjugates of specific antibodies and nanoparticles are used in the tests to include colored labels into immune complexes, and the most common nano labels among them are Gold Nanoparticles (GNPs) [11, 12].
1.2. Principle of Common Competitive Immunochromatography
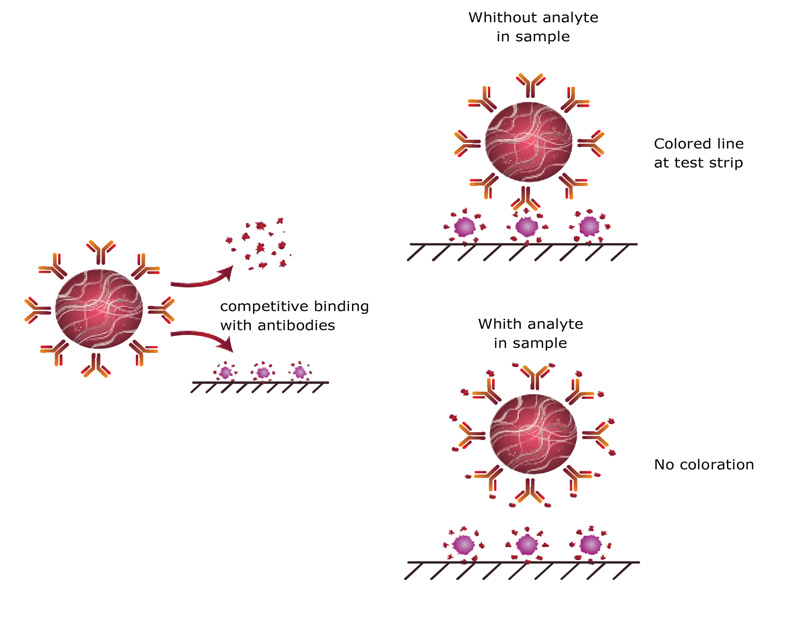
Low molecular weight analytes with only one epitope for antibody binding can be detected only by competitive schemes of immunoassays. In traditional competitive ICA, GNPs are conjugated with antibodies specific to an analyte and this conjugate is applied on a membrane of a test strip [10]. If the analyte is absent in the sample, the conjugate comes to the zone with an immobilized antigen and binds there with the formation of colored line. If the content of the analyte in the sample overcomes its controlled (cut-off) level, it blocks binding sites of the antibodies and does not allow them to interact with an immobilized antigen (Fig. 1). Non-bound conjugate comes to the next zone at the test strip with immobilized anti-species antibodies and forms a colored line here. The distribution of GNP-labeled antibodies between the free analyte in the sample and the antigen immobilized on the test strip indicates the analyte content. The higher the content of the detected antigen, the lesser the GNP-antibody conjugate is bound to the membrane and the weaker is the staining of the corresponding zone of the test strip.
1.3. Limitations of Common Competitive ImmunoChromatography
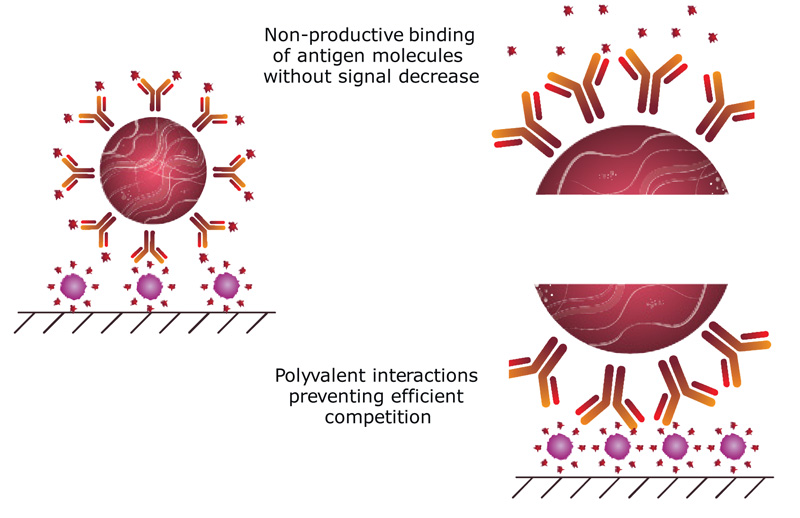
However, real competitive ICA is associated with several problems limiting the sensitivity of the analyte’s detection. To reduce the detection limit, it is necessary to reduce the concentration of specific antibodies, but this leads to a decrease in the intensity of the color band formed, and makes the detection process difficult. Moreover, some additional factors also influence the sensitivity and make it difficult to achieve high analytical parameters in competitive ICA. Firstly, the high density of specific antibodies immobilized on the surface of a nanoparticle leads to the fact that the binding of most of them with the free antigen does not prevent from the binding of the conjugate to the immobilized antigen (non-productive binding, Fig. 2). Secondly, possible polyvalent interactions between the nanoparticle-antibody conjugate and the immobilized antigen prevent the free antigen with lower affinity of monovalent interaction from competitively displacing the conjugate from the membrane (Fig. 2) [13, 14].
1.4. Competitive Immunochromatography with Indirect Labeling
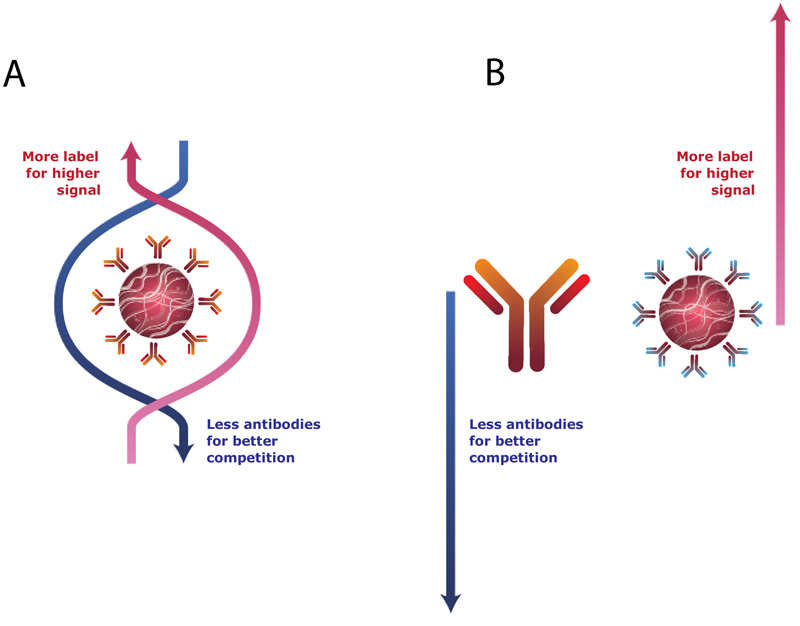
Thus, the label (GNP) content in the system should be high to reach an intense signal, and the antibodies content should be low for effective competition. These demands are contradictory if they are addressed to the same preparation, the use of conjugated complex between gold nanoparticles and specific antibodies (Fig. 3A). To overcome this limitation, we have earlier proposed an indirect labeling approach for use in ICA [13, 15, 16]. This approach increases the assay sensitivity and, at the same time, its rapidity and simplicity of implementation. The commonly used (specific antibody – GNP) conjugate is replaced by a combination of free, non-conjugated specific antibodies and anti-species antibodies conjugated with GNPs (Fig. 3B). In this indirect labeling, all the interactions of antibodies with the antigen contained in the sample lead to a decrease in label binding, which shifts the limit of detection and the working range of the assay to lower concentrations. The effectiveness of this approach was shown for several mycotoxins, beta-agonists and antibiotics [15-26].
This publication provides a comparative assessment of the various options of indirect labeling in ICA based on the presented experimental data for two important food contaminants, aflatoxin B1 (AFB1) and T-2 toxin (T2T). The necessity of wide screening of their contamination is due to widespread distribution and significant multiple toxic effects of these mycotoxins [27-29]. The assays were realized using gold nanoparticles as the most common labels in immunochromatographic tests and commercial monoclonal antibodies against T2T and AFB1 having high affinity and not containing inactive molecules, unlike polyclonal antibodies. The presented data are compared with the previous realization of ICA with indirect labeling, and common conclusions about its possible formats are given.
2. MATERIALS AND METHODS
2.1. Materials
T-2 toxin (T2T) and aflatoxin B1 (AFB1) were purchased from Chromresurs (Moscow, Russia). Mouse monoclonal antibodies against T2T and AFB1 were provided by IL-TEST Pushchino (Pushchino, Moscow region, Russia). T2T-BSA conjugates were purchased from BioTez Berlin-Buch (Berlin, Germany). Goat anti-mouse polyclonal antibodies were obtained from Arista Biologicals (Allentown, PA, USA). Sodium azide, Triton X-100, Tween-20, and chloroauric acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bovine Serum Albumin (BSA) was purchased from MP Biomedicals (Santa Ana, CA, USA). The purity of all other reagents was of analytical grade or higher.
2.2. Preparation of Gold Nanoparticles
Gold Nanoparticles (GNPs) with an average diameter of 30 nm were synthesized as described earlier [30]. Briefly, 1.0 mL of a 1% water solution of HAuCl4 was added to 97.5 mL of water. The mixture was heated to reflux, and 1.5 mL of 1% sodium citrate solution was added. After refluxing for 30 min, the preparation was cooled and then stored at 4°C.
2.3. Immobilization of the Antibodies on the GNPs
The pH of the GNP solution was adjusted to 8.5-9.0 with potassium carbonate. Monoclonal antibodies against AFB1 or T2T, or goat anti-mouse polyclonal antibodies, were converted into 10 mM Tris buffer solution, with pH 9.0, and added to the GNP solution (from 0.5 to 20 μg per mL of the GNP solution). The resulting mixture was incubated for 45 min at room temperature, followed by the addition of a 10% aqueous solution of BSA (VGNP:VBSA = 40:1) and vigorous stirring for 15 min. The GNPs were pelleted by centrifugation at 13000 g for 15 min at 4 °C. The supernatant was removed, and the residue was dissolved in 10 mM Tris buffer, pH 7.5, with 1% BSA, 1% sucrose and 0.05% sodium azide (TBSA) and stored at 4°C.
2.4. Production of Immunochromatographic Tests
The test strips included Hi-Flow Plus immunochromatographic membranes (Millipore, Billerica, MA, USA, merckmillipore.com). РТ-R7 glass fiber pad and a Р045 adsorption pad were obtained from (MDI Easypack, Advanced Microdevices, Ambala Cantt, India, www.mdimembrane.com). The completion of test systems may be varied (see 2.5) for specific descriptions.
Reagents were immobilized on the membranes using an Iso-Flow automated dispenser (Imagene Technology, Hanover, NH, USA, imagenetechnology.com) at a rate of 0.1 µL per mm. After dispensing, the membrane was dried at room temperature for at least 24 h. The load of the conjugate of GNPs and antibodies was 3.2 µL for 1 mm of glass fiber membrane width. The glass fiber membrane was dried at room temperature for at least 24 h. The working and terminal absorbent pads were fixed on a plastic pad.
After the assembly of the membrane components, the obtained sheets were cut with Index Cutter-1 (A-Point Technologies, Gibbstown, NJ, USA) into test strips of 3.5 mm in width and stored at 20–22 °C in a sealed package containing silica gel.
2.5. ICA Procedures
2.5.1. Common Demands
Samples were spiked with mycotoxins at concentrations of 0.01–10.0 ng/mL. The assay was carried out at room temperature. Each sample was tested in triplicate.
2.5.2. ICA with Direct Labeling
Tests Preparation: The test zone was formed by the AFB1-BSA or Т2Т-BSA conjugates (1 mg/mL in 50 mM phosphate buffer solution, pH 7.4, with 0.1 M NaCl (PBS)). The control zone was formed by the goat anti-mouse IgG (0.5-1 mg/mL in PBS). The conjugate of GNPs and anti-mycotoxin antibodies was spotted onto a glass fiber membrane at a dilution corresponding to D520 = 1.0.
Assay: The test strip was vertically submerged into a mycotoxin-containing sample for 10 min, removed and placed on a horizontal surface. After 5 min, the assay results were recorded.
2.5.3. Stepwise ICA with Indirect Labeling
Tests preparation: The test zone was formed by the AFB1-BSA or Т2Т-BSA conjugate (1 mg/mL in PBS). The test strips did not contain a fiberglass membrane and sample pad.
Assay: Dilutions of AFB1 or T2T were prepared in the range from 10 to 0.01 ng/mL and solutions of anti-mycotoxin antibodies (300 ng/mL) were added and incubated for 5 min after which, the test strips were immersed and incubated for additional 5 min. Then, the test strips were transferred to wells containing 100 μL of GNPs conjugated with anti-species antibodies (D520 = 1.0). After 10 min, the assay results were recorded.
2.5.4. ICA with Indirect Labeling when all Reagents are Applied to the Test Strip
Tests preparation: The test zone was formed by the AFB1-BSA or Т2Т-BSA conjugate (1 mg/mL in PBS). The conjugate of GNPs with anti-species antibodies at a dilution corresponding to D520 = 3.0 and anti-mycotoxin antibodies (300 ng/mL) were spotted onto a glass fiber membrane.
Assay: The test strip was vertically submerged into a sample for 10 min, removed and placed on a horizontal surface. After 5 min, the assay results were recorded.
2.5.5. ICA with Indirect Labeling when Antibodies are in Solution
Tests Preparation: The test zone was formed by the AFB1-BSA or Т2Т-BSA conjugate (1 mg/mL in PBS). The conjugate of GNPs with anti-species antibodies at a dilution corresponding to D520 = 3.0 was spotted onto a glass fiber membrane.
Assay: Dilutions of AFB1 or T2T were prepared in the range from 10 to 0.01 ng/mL and solutions of anti-mycotoxin antibodies (50 ng/mL) were added and incubated for 3 min after which the test strips were immersed and incubated for additional 10 min.
2.6. Registration and Processing of ICA Data
The binding of the label in the test and control zones was recorded by a CanoScan LiDE 90 scanner (Canon, Tokyo, Japan), followed by the digital processing of the images with TotalLab software (Nonlinear Dynamics, Newcastle, UK). This program was used to sum up the intensities of all pixels belonging to a particular binding zone, and normalize the sum to the surface area, thereby representing the color intensity in relative units.
Based on the registered color intensities (Y) of various concentrations of the analyte (x), a calibration curve was plotted using the four-parameter sigmoid function:
y = (A – B) / (1 + (x/C)D) + B,
where x is the analyte concentration, y is the color intensity, A is the asymptotic maximum of the color intensity, B is the asymptotic minimum (background value) of the color intensity, C is the inflection point of the curve in the semi-logarithmic coordinates (equal to 50% inhibition of the color intensity), and D is the slope of the curve at the inflection point.
The visual limit of detection was interpreted as the minimal concentration of toxins that caused the complete absence of coloration in the test zone (i.e., its coincidence in color with neighboring regions of the strip). The quantitative limit of detection was calculated as the toxins content corresponding to 10% binding inhibition.
3. RESULTS AND DISCUSSION
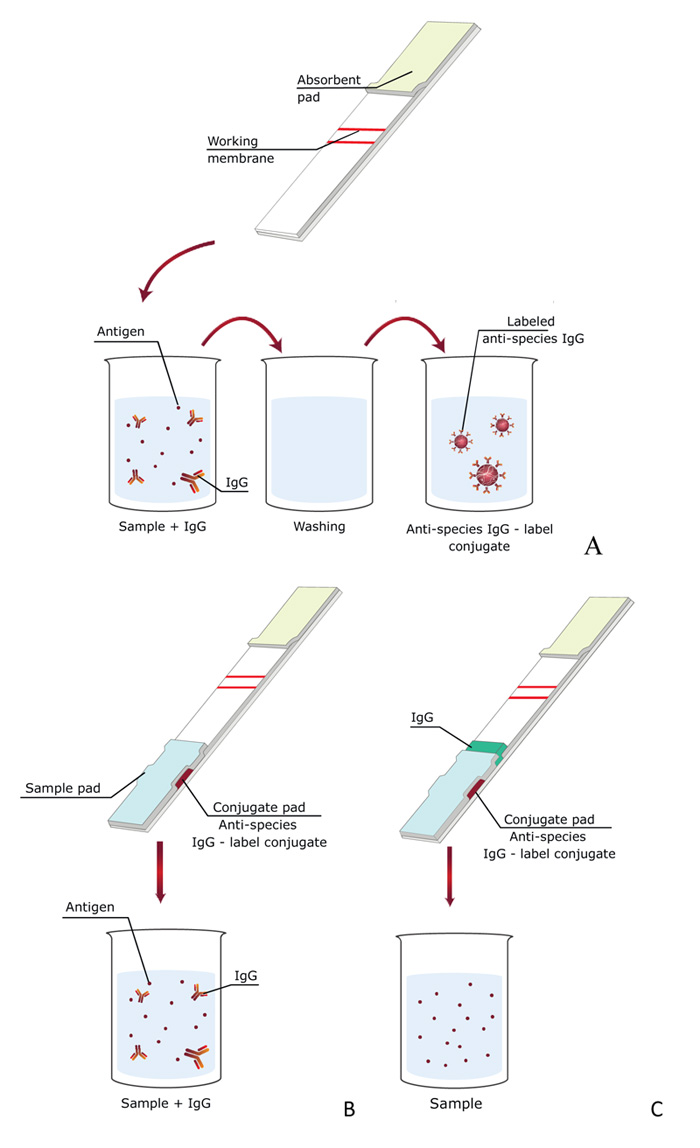
The proposed concept of indirect labeling in ICA could be realized via three schemes that differ in the location of reactants used to form the detectable complexes. These schemes are presented in Fig. (4).
3.1. Stepwise ICA
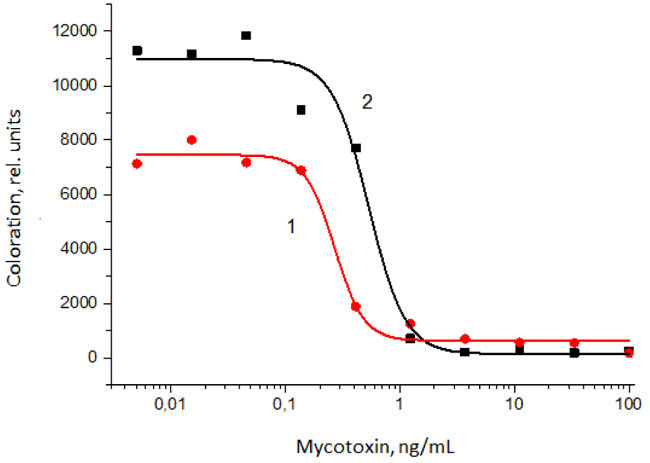
In this scheme [13], three liquid reagents are successively passed along the test strip: specific antibodies with a sample, wash buffer, and the conjugate of GNPs with anti-species antibodies (Fig. 4A). Thus, this analysis scheme consisted of three successive stages: (i) competitive interaction of specific antibodies with the antigen of the analyzed sample and antigen immobilized on the test strip, (ii) washing with buffer to remove unreacted components, (iii) interaction with conjugate of GNPs with anti-species antibodies.
For the stepwise ICA, the concentration of the marker does not affect the competitive interactions occurring in the analytical zone. Therefore, the concentration of conjugate of GNPs with anti-species antibodies was taken in excess. The reduction in the content of specific antibodies with a significant increase in the number of markers allowed for a competitive interaction with a low detection limit and high coloration.
To realize this approach for ICA with indirect labeling, the assays for AFB1 and T2T have been developed and optimized. The obtained calibration curves of the assays are presented in Fig. (5). As can be seen, in the case of visual detection, the detection limits are 1 and 0.5 ng/mL for AFB1 and T2T, respectively. Instrumental detection limits for the developed assays are 0.14 and 0.24 ng/mL, respectively. Compared to the direct scheme of ICA, the visual detection limits decreased by 20 times for both the analytes. The ICA allowed detecting AFB1 and T2T in methanol:water (7:3) extracts of cereals without loss of sensitivity.
3.2. ICA when all Reagents are Applied to the Test Strip
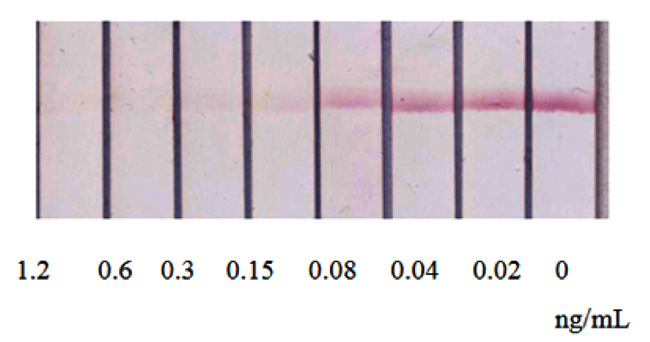
Due to increasing number of stages and long time of analysis, an alternate scheme in which all immunoreagents were applied on a test strip was proposed [31]. The test strip consists of several membranes located on a plastic surface: separation membrane, two glass fiber membranes, a working nitrocellulose membrane on which a detectable colored line is formed, and a final absorbing membrane that provides fluid flow along the text strip. The specific feature of the proposed test strips is the use of two fiberglass membranes instead of one. One membrane contains a GNPs’ conjugate with anti-species antibodies. This membrane is shifted closer to the bottom of the test strip and is not in direct contact with the working membrane. The second one, located above, contains free (unmodified) anti-mycotoxin antibodies (Fig. 4C).
The contact of the test strip with the sample leads to the movement of fluid along the membranes and the interaction of specific antibodies, first with the antigen in the sample, and then with the immobilized antigen-protein conjugate. The marker conjugate with anti-species antibodies later leaves the corresponding membrane due to the geometry of the location and the greater mass of the conjugate as compared to free antibodies, reaches the analytical zone and binds in it to the protein-antigen-specific antibody complexes, ensuring its staining.
The given ICA of AFB1, realized under optimized conditions, was characterized by a detection limit of visual and instrument detection of 0.6 ng/mL and 0.04 ng/mL, respectively (Fig. 6). Thus, the reduction in the assay duration was accomplished bysome (2-3-fold) decrease in the detection limit in comparison with the first assay format.
3.3. ICA when Antibodies are in Solution
The movement of the antigen and antibodies along the test strip in ICA occurs in a laminar mode, as a result of which, the probability of a collision and subsequent binding decreases relative to the free diffusion of the same components in the solution. That is why the efficient approach to lowering the detection limit in ICA is to preincubate the GNPs-specific antibodies conjugate with a sample containing the antigen.
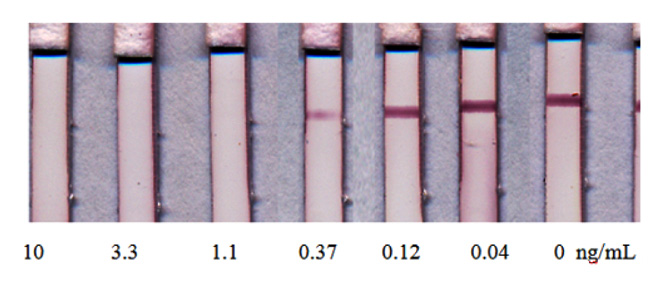
The corresponding ICA with indirect labeling was also considered [16]. The dry test strip already contains all the components (membranes, GNPs conjugated with anti-species antibodies and conjugate of antigen with protein), but anti-mycotoxin antibodies are transferred to the buffer for diluting the tested sample (Fig. 4B). Thus, the procedure for determining the antigen does not differ from the traditional scheme but provides both the preincubation of antibodies and indirect labeling.
However, the realization of this assay format for T2T was not accomplished by additional improvements in analytical parameters as compared with the initial (Fig. 4A) scheme. The ICA of T2T was characterized by visual and instrument detection limits of 1.1 ng/mL and 0.12 ng/mL, respectively (Fig. 7). The main reached advantage of the tested scheme was the reduction of assay duration.
3.4. Comparing Three Formats of ICA with Indirect Labeling
The obtained experimental data allow summarizing advantages and limitations of the three considered formats of ICA with indirect labeling. The corresponding generalization of the present and previous studies is given in Table 1.
| Formats | Stepwise ICA | ICA when all Reagents are applied to the Test Strip | ICA when Antibodies are in Solution |
|---|---|---|---|
| Advantages | Assays steps do not affect each other | Minimal limit of detection | Pre-incubation is combined with sample dilution |
| Limitations | Longer assay More laborious manipulations |
Influence of the test strip geometry and the mode of reagents deposition on the assay results | Loss of sensitivity |
CONCLUSION
The proposed schemes for ICA clearly demonstrate that the separation of the stage of specific interaction and the stage of introduction of the labeled conjugate in ICA allows reducing the concentration of specific antibodies and increasing the sensitivity of the analysis. It was shown that indirect labeling in the analysis of mycotoxins made it possible to increase the sensitivity up to 20 times compared with the traditional scheme. The presented data are in accordance with previously presented comparisons of ICA with direct and indirect labeling where the decrease of the detection limit varied from 5 times to 3 orders of magnitude [15-26].
Thus, the proposed assay formats have minimal demerits due to a more complex set of analytical kit. However, these assays are characterized by a reduction in the consumption of specific antibodies and the use of conjugates of anti-species antibodies with GNPs, which are universal for mono- or polyclonal antibodies and can be introduced into test systems for detecting various analytes.
The presented studies allow recommending the indirect immunochromatography for more sensitive assays of other low molecular weight analytes that are controlled in medical diagnostics, food safety and environmental control.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This work was supported by the Russian Foundation for Basic Research, project # 18-53-18013.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


