All published articles of this journal are available on ScienceDirect.
An Easy Method for Screening and Detection of Laccase Activity
Abstract
Objective:
An instrument-free assay was developed for simultaneous detection of laccase activity in a large number of samples as diverse as screening of laccase-producing microbial cultures or chromatographic fractions.
Method:
Dried paper discs previously impregnated with 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) were placed on a flat-bottom microplate (a simple way to avoid misidentification) and loaded with an aliquot from each sample.
Results:
Discs corresponding to samples containing laccase activity become green-bluish colored within first ten minutes of reaction, allowing direct detection through simple naked-eye inspection.
Conclusion:
As an example, this easy process was applied to the laccase purification in order to eliminate chromatographic fractions that did not contain laccase activity, thus reducing the number of spectrophotometric assays.
1. INTRODUCTION
Laccases (benzenediol: oxygen oxidoreductase, EC 1.10.3.2) and laccase-like metalloxidases [1] are glycoenzymes that belong to the family of multicopper oxidases. They catalyze non-specific oxidation of natural or synthetic substrates such as phenolic or non-phenolic aromatic compounds, amines and inorganic ions or molecules, with a concomitant four-electron reduction of molecular oxygen to water. In recent years, these enzymes have received increasing attention due to their potential for wide-ranging biotechnological applications [2, 3] including environmental pollution abatement [4, 5]. As a consequence, a growing number of laccases from diverse organisms (filamentous fungi, yeasts, plants, bacteria, and even insects) but mainly from species belonging to the ecological group of white-rot basidiomycetes have been characterized at biochemical and molecular levels [2, 6].
As a rule of thumb, a process of isolation and purification or at least partially enzyme purification [7] should be done before proceeding with detailed structure-function characterization including determination of kinetic parameters. More than one chromatographic step is usually performed during purification procedures in order to obtain an enzyme-enriched preparation free of other proteins or contaminant compounds such as inhibitors. In general, chromatographic processes yield a large number of fractions that have to be analyzed for the presence of enzyme activity, usually by spectrophotometry. The method described here is a simple screening procedure that enables rapid detection of laccase-containing samples. In this work, its usefulness is illustrated, but not limited, to detection of laccase activity in chromatographic fractions during purification of laccase produced by the white-rot fungus Ganoderma resinaceum.
2. MATERIALS AND METHODS
2.1. Production and Purification of Fungal Laccase
The fungal strain G. resinaceum Ga-20, from the culture collection at Laboratory of Agro-Environmental Technologies of UTAD, was grown in Potato Dextrose Agar (PDA) plates and incubated at 28°C for six days. As described earlier [8], Erlenmeyer flasks containing 100 ml of liquid medium supplemented with 5% (v/v) olive mill wastewater (whose main characteristics were as follows: 0.8 g phenols l-1, 12.6 g total solids l-1, chemical oxygen demand 53.5 g O2 l-1) were inoculated with two 1 cm2 agar plugs removed from fungal PDA plates and incubated at 28ºC and 120 rpm.
Culture medium was harvested at 12th day of incubation, freeze-thawed, filtered (Whatman GF/A) and simultaneously equilibrated with 15 mM Tris-HCl pH 7.0 (buffer A) and concentrated by ultrafiltration performed under nitrogen at a positive pressure of 3.5 bar in an Amicon stirred cells unit fitted with a 10 kDa cut-off membrane. Subsequent anion exchange chromatography of concentrated crude laccase was accomplished in a DEAE-Sepharose FF column (10 x 150 mm) connected to a Gilson system (two peristaltic pumps and dynamic mixer, UV/Vis detector, and fraction collector). After baseline stabilization, bound proteins were eluted at a flow rate of 1.6 ml min-1 with the following gradient of buffer B (15 mM Tris-HCl pH 7.0; 1 M NaCl): t = 0 min, %B = 0; t = 20 min, %B = 0; t = 54 min, %B = 50; t = 58 min, %B = 100; t = 60 min, %B = 100; t = 61 min, %B = 0. The fraction collector was set to change fraction tubes at 2-min intervals, which means a total of 30 fractions.
2.2. Easy Assay for Rapid Screening of Laccase Activity
2.2.1. Materials and Its Preparation
Standard 6 mm blank paper discs (BBL; Becton Dickinson & Company) were soaked for 2 min in a beaker with a solution of 2 mM 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) prepared in 100 mM phosphate-citrate buffer pH 4.0. Subsequently, ABTS-wet discs were removed with the aid of a simple colander and dried in an oven at 60°C. After cooling, dried ABTS-impregnated discs should be stored in a sealable glass or plastic flask in the refrigerator until use.
2.2.2. Methodology
In this work, 10 dried ABTS-impregnated discs per each row were placed into an empty standard flat-bottom 96-well microplate. The screening of laccase activity was started by the addition of 10 µl aliquots from each sample (in this work, chromatography fractions) to discs. Within the first 10 min of incubation at 30ºC, discs loaded with aliquots from chromatography fractions containing laccase activity will present a green-bluish color development, easily visible by simple naked eye inspection.
2.3. Determination of Laccase Activity
Spectrophotometric determination of laccase activity was carried out at 25ºC by measuring the oxidation of 2 mM ABTS in 100 mM phosphate-citrate buffer pH 4.0. The formation of cation radical ABTS• + was followed by absorbance measured at 420 nm (ε420 = 36 mM-1 cm-1) using an UV-Vis spectrophotometer [9]. One enzyme unit (U) is defined as the amount of enzyme which oxidizes 1 µmol of substrate per minute in the assay conditions.
3. RESULTS AND DISCUSSION
The laccase activity in culture media fermented by the white-rot fungus used in this work (G. resinaceum) or other laccase-producing white-rot fungal strains, such as Trametes versicolor, Phlebia rufa and Fomes fomentarius, are routinely detected in our laboratory using the proposed easy assay for rapid screening of laccase activity. Thus, the use of G. resinaceum in this study “per se” is not important or relevant to this work, since it was only used as a laccase-producing strain.
In this work, taking into account the chromatographic runtime and the fact that the fraction collector was set to collect and change the tubes at every two minutes intervals we have about 30 fractions per each chromatographic run that need to be analyzed for the presence of laccase activity. In order to cut down the number of spectrophotometric measurements of laccase activity, a simple zymogram assay was developed which relies on an equipment-free combination of two products readily available in most laboratories: blank paper discs (previously impregnated with ABTS) and microplates.
The upper panel of Fig. (1) shows the results of the proposed zymogram assay (color development of ABTS-impregnated paper discs) and the lower panel of Fig. (1) shows the plot of laccase activity of selected corresponding chromatographic fractions measured by the spectrophotometric assay (fractions number 2, 7, 10, 27 and 29 were not measured by spectrophotometry). As can be seen, most of the laccase activity occurs between fractions 19 - 21 as detected by the proposed zymogram assay. In fact, the peak of laccase activity was eluted with a saline gradient at about 0.28 M NaCl and was recovered in fraction number 20 (elution time between 38 - 40 min). Since this result matches the spectrophotometric determinations, proves that the zymogram assay is a useful, fast and efficient screening procedure.
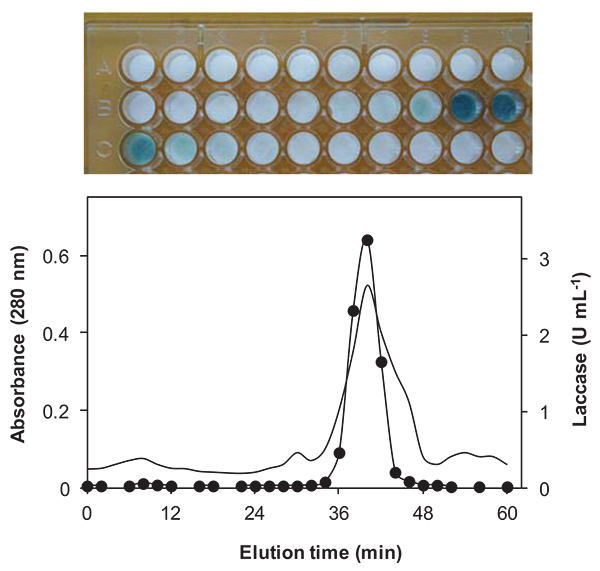
Diverse techniques have been used for screening of extracellular lignocellulolytic enzymes and for detection of laccase in purified samples [10-12]. The proposed method described here, present the following main advantages: i) blank paper discs commonly used for compound sensitivity tests were chosen due to its appropriated dimensions such as thickness and friendly handling; ii) paper discs were impregnated with a diluted ABTS solution (2 mM) but it should be buffered at an acidic pH (e.g. 4.0); iii) the use of a microplate as a support for the ABTS-impregnated discs, also is a simple way to match each disc to a given fraction, thus avoiding sample misidentification.
The use of a buffered substrate solution is a critical point that needs particular emphasis because using paper discs impregnated with non-buffered ABTS solution can lead to false negative results. This is due to a monotonic decrease of laccase activity as the reaction pH value increases, typically observed in the pH-activity profile using ABTS as substrate [13]. In fact, a pH between 3.0- 4.0 is the optimal reaction value for the oxidation of ABTS catalyzed by most fungal and bacterial laccases [13-15]. Finally, dried paper discs impregnated with substrate can be stored in a refrigerator for long time, ready to be used when needed (according to our own experience no activity loss was observed after six months storage).
CONCLUSION
A new zymogram assay was applied to the detection of laccase activity. As a concluding remark, the method proposed here is a simple and economic process that will greatly cut down on tedious and unnecessary spectrophotometric assays of laccase activity during the screening of large number of samples as is the case of purification steps or detection of laccase activity in fermentation broths.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


