All published articles of this journal are available on ScienceDirect.
Micellar Enzymology- Chemistry and Applications
Abstract
Enzymes in aqueous environment usually deal with purified enzyme preparations isolated from living matter which does not mimic real catalytic properties in vivo. Interaction of enzymes in nature takes place with different surfaces composed from lipid membranes or they get incorporated into biomembranes. Although Water is not a dominating component in the cytoplasm but plays a structural role by participating in the formation of biocatalytic complexes like glycoproteins. Water is needed to keep biocatalyst in active confirmation and hence plays very crucial role in biocatalytic reactions, activity and stability so that it can be used for various applications. This review focuses on composition, preparation properties and parameters which influence enzymes in reverse micelles and application of micellar enzymology to study protein chemistry, shifting equilibrium of various reactions, to recover various products by partition chromatography and bioremediation of chlorophenolic environmental pollutants.
1. INTRODUCTION
In molecular enzymology, mainly free enzymes are studied, where the main focus is on elucidating the structure of catalytic centers and physico-chemical mechanisms of biocatalysis. These experiments were successful only with the enzymes isolated from the living cells in a pure form. However, experiments with such ‘native’ enzymes imposed the question regarding the correlation of enzyme properties and functions observed in vitro and in vivo conditions. Such doubt becomes quite evident as it is clear that the subcellular structure and the compartmentalization of enzymes play the most important role in the regulation of metabolism [1].
‘Micellar Enzymology’ named by Martinek's group, emerged in the late 1970s, studied the self-organizing properties of amphiphiles in solution called ‘Reverse Micelles’ which mimic the microenvironment that enzyme finds in the cell [2]. Three major components: amphiphilic surfactant molecules, water and non-polar organic solvent constitute reverse micelles system in which polar heads of the surfactant molecules are projected towards the interior of a water containing sphere, whereas the aliphatic tails are facing towards the non-polar organic phase [1]. The water structure within the reverse micelles mimics water content adjacent to biological membranes [3].
2. COMPOSITION AND PROPERTIES OF REVERSE MICELLES
Reverse micelles are water-in-oil emulsion which is formed by adding surfactants in organic solvent, and nanometer sized water pools, formed by the solubilization of water in their polar cores. Mostly such ternary complex is spherical in shape and composed of <10% of surfactant, 0-10% of water and 80-90% of organic solvent. Such complexes have an aggregation number <50 which is smaller than their hydrophilic counterparts called micelles. Reverse micelles do not undergo phase separation with time as they are thermodynamically stable due to their colloidal property. They can also undergo spontaneous formation and have low interfacial tension [<10-2 mN·m-1]. They are also transparent in nature due to <100 nm size hence provide a large surface area of 102-103 m2·cm-3. Such preparation has almost similar viscosity as pure organic solvents which constantly collide and fuse with each other, occasionally, the fused surfactant molecule and the content inside reverse micelles undergo exchange [4].
Reverse micelles are ideal system for enzymological studies because of their macroscopic properties like thermostability, optical transperancy and capacity to accommodate a large amount of host molecules and hence techniques such as CD (Circular Dichromism), polarization and time resolved fluorescence, phosphorescence, UV visible spectroscopy and dynamic light scattering spectroscopy can be easily employed [2].
3. REVERSE MICELLES FOR PROTEINS AND ENZYMES
There are three major factors which influence enzyme activity in reverse micelles systems.
- The most important factor that regulates enzymes and proteins in the reverse micelles is the geometrical factor. However, maximum enzyme activity is obtained when protein fits properly into the size of reverse micelles [5].
- The hydration ratio W0 = [H20]/[Surfactant] influences the size of the inner diameter of the cavity and hence the compressibility of biocatalyst in the reverse micelles. Micellar size can also be increased by increasing the water content, when both water and surfactants concentrations are increased simultaneously, it will also increase the reverse micelles concentration, while increase in surfactant concentration decreases micelles size.
- Type of solvent used for the preparation of reverse micells influences both the water activity and compressibility of protein in the water pool. Since non polar solvents reduce the product inhibition, confined the biocatalyst to the aqueous phase and increase its thermostability in contrast to polar organic solvents which are preferred for the preparation of reverse micelles [6, 7].
4. MECHANISM OF ACTION
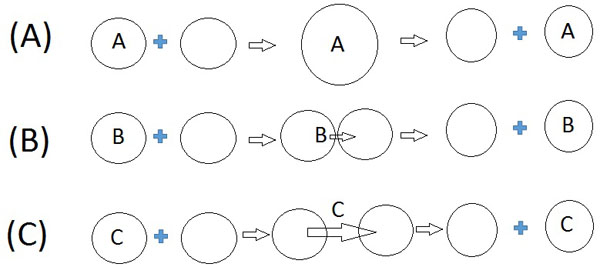
Electrolytes and proteins solubilized in the water pools of reverse micelles are exchanged between reverse micelles. The possible mechanisms proposed for these solubilisate exchange processes are shown in Fig. (1). Mechanism A involves the transient fusion of two reverse micelles to form a short-lived 'dimer droplet', the solubilized molecules can then redistribute through diffusion during the finite lifetime of this dimer, the 'dimer droplet' splits into two reverse micelles. Mechanism B involves the diffusion of the exchanging molecules through the surfactant bilayer formed at the point of contact of non-fusing reverse micelles. Mechanism C involves migration of the solubilisates through the oil/solvent phase [8-10].
5. DECLARED PARAMETERS TO BE CONSIDERED FOR THE PREPARATION OF REVERSE MICELLES SYSTEM
5.1. Effect of Organic Solvents
Different researchers evaluated the role of different organic solvents on the AOT [Bis(2-ethylhexyl) sulfosuccinate sodium salt] based laccase/RM complex. Isooctane was found to promote the highest activity of different proteins similar to the activity of protein in the aqueous medium. One of the reasons might be the structural similarity between isooctane and the tail portion of AOT which provides the best penetration of isooctane into AOT tails [11]. Water miscible solvents are not preferred because of their low logPoctanol values which are a measure of hydrophobicity of solvent. logPoctanol values are defined as logarithm of the partition coefficient of the solvent in a standard two phase system of 1-octanol and water. Isooctane is more biocompatible for cellular biocatalyst as its logPoctanol value is > 5 [5, 12-14].
5.2. Physicochemical Properties of the Proteins
Proteins hosted in the reverse micelles exhibit ‘super activity’ because of tight packing of amino acid residues at the internal cavities which provide them better compressibility and volume changes as compared to the aqueous environment [15]. In such type of system, large amount of water can be accommodated inside the voids which act as a structural stabilizer by maintaining good hydrogen bond between the domains and filling sites of imperfect packing [16]. In contrast to that water interacting at the protein surface negatively contributes to the protein volume and compressibility [17].
5.3. Effect of Surfactant Concentration and Hydration Ratio [W0]
Surfactant concentration play a crucial role in order to obtain high protein activity in the reverse micelles, because reaction proceeds by the collision and fusion of RMs (Reverse Micelles). Very low concentration of surfactant < 150 m. M increases the water pool of reverse micelles which reduces an overall affinity of enzyme towards its substrate by increasing the distance between them [18]. On the other hand, a very high concentration leads to electrostatic repulsion and leads to denaturation of the protein. The concentration of surfactant also influences the water activity of the reverse micelles which can be quantitatively expressed as hydration ratio Wo, one of the important parameters of water pool in the reversed micelles and expressed as [H2O]/ [surfactant]. There is a linear relationship between the size of the reversed micelle and available water pool [19]. The optimum Wo value for any protein is determined by the size of the protein because appropriate space is required to encompass the protein as it determines the acquired conformation inside the micelle [20]. When the size of the inner cavity matches the size of the enzyme molecule, the maximum catalytic activity is observed. The range of Wo which promotes reverse micelles formation, ranges from 15 to 30. It has been observed by different researchers that values below 15 lead to direct interaction between the hazardous organic solvent and enzyme while, higher values above 30 generate large water pool which cannot accommodate the enzymes. There is a bell-shaped relationship between the catalytic activity of the enzyme and hydration ratio with respect to most of the enzymes studied in reversed micelles. However, two optimum values were reported by Okazaki et al. who considered oligomeric nature of the protein with a different molecular size [19].
5.4. Effect of Temperature
Thermostability of proteins/enzymes hosted in reverse micelles can be explained by considering the fact that water is one of the reactants in thermo-inactivation processes which decreases the rigidity of the protein. As a consequence, there will be a reversible thermo-unfolding; heat induced incorrect structure formation and aggregation of enzymes. As compared to aqueous phase, the rigidity of enzymes/proteins structure in non-polar organic solvents is well maintained, which explains the thermostability of enzymes in dry organic solvents [21]. Thermostability of chymotrypsin [22], terpene cyclase [23], ATPase and cytochrome oxidase [24] was found to increase in the presence of hydrophobic solvent.
6. METHODS OF PROTEIN HOSTING IN REVERSE MICELLES
Preparation of reverse micelles for proteins/enzymes using surfactant and organic solvents is carried out by following methods.
According to the “injection method’, small amount of aqueous solution of protein is injected into the surfactant solution in a non-polar organic solvent. A ratio of water and organic solutions is defined by the conditions of the experiment, primarily, by the value of the desired degree of hydration ratio. After injection, the obtained mixture is shaken vigorously for few seconds until an optically transparent solution is formed [25].
Menger and Yamada [26] proposed the second method in which, a suitable amount of aqueous buffer solution was first introduced into the solution of surfactant in an organic solvent in order to achieve the desired value for the degree of hydration ratio; followed by the dissolution of lyophilized protein preparation in the micellar solution by vigorously shaking the mixture. This procedure takes few minutes to few hours for solubilization than in the case of solubilization of aqueous solutions as mentioned in the first method. This procedure may lead to protein denaturation because of its contact with the surfactant and the organic solvent for relatively longer time. However, by this method micelles can be packaged with protein having high concentration than the simple injection method.
Protein is spontaneous distributed in a biphasic system consisting of nearly equal volumes of aqueous protein solution and surfactant containing organic solvent is the basis of the third method for reverse micelles preparation. In this method protein transfer takes place with or without slight stirring and lasts for a relatively longer duration. This procedure allows better interaction between protein [enzyme] surfactant and organic solvent in the aqueous solution but the concentration of protein at the interface may lead to denaturation of protein, which is disadvantageous over those methods described earlier [27].
Because of the microheterogenous nature of reverse micelles system, isolation of components from these systems is quite a difficult task and requires an individual consideration. However, recovery of proteins and the products of the reactants from the reverse micelles can be achieved by temperature shift, using semipermeable membrane and by adding water miscible organic solvents like acetone or ethanol [28].
7. STRUCTURAL ASPECTS OF PROTEIN-CONTAINING MICELLES
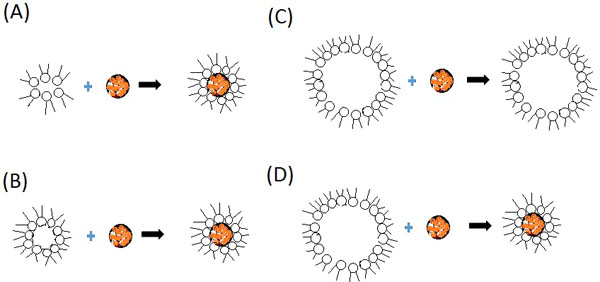
Reverse micelles which are based on isooctane and AOT, there is a linear relationship between radius of the micelles and the degree of hydration [water-to-AOT molar ratio], W0=[H2O]/[AOT]: rM=4+1.5 wo [in A˚]. The protein having different size can be packaged inside the cavity of the micelle by changing the hydration ratio. A schematic representation of different examples of protein-containing micelles is shown in Fig. (2). If the micelles are smaller in size then there a new micelle is created out of a few micelles so that in a protein-containing micelle, the protein molecule becomes surrounded by a surfactant monolayer [induced fit model] (Fig. 2a). The size of the protein and the length of the surfactant molecule decide size of the micelle. In a fixed size model, hydrophilic proteins convolute to form inner cavity of large micelles without changing the external sizes of hydrated micelles (Fig. 2c). This model also explains the working of large where the radius of the inner cavity of the micelle is equal to the protein sizes (Fig. 2b). Water in this case forms the inner cavity of the formed micelles shifts towards carbohydrate-containing surfactant chains. The “induced fit” mechanism is adopted by Membrane-sensitive enzymes when interact with the micellar matrix and form an optimal micellar shell (Fig. 2d) [29, 30].
8. KINETIC CHARACTERISTICS OF ENZYMATIC CATALYSIS IN SYSTEMS OF REVERSE MICELLES AND THEIR REGULATION
Due to the microheterogenous system of reverse micelles, it is important to define their local concentration which can be attributed to the distribution of the reagents in the reverse micelles. When the determination of kinetics parameters like Kcat and Km of enzyme is carried out and since the expression of Km is in the units of concentration, its analysis becomes difficult. The Km value is influenced by the distribution coefficient of the substrates and the volume ratio of the phases. Different types of multi-phasic theoretical models and experimental approaches are considered for the interpretation of results for reverse micelles [18, 20, 28, 31, 32]. The first order rate constant kcat [Sec]-1 is reflecting a true reactivity of enzyme in the reverse micelles because this kinetic parameter is not complicated by the distribution effects of the substances and may be regarded as an objective parameter.
9. APPLICATIONS OF MICELLAR ENZYMOLOGY
Enzymes or proteins hosted in ternary micellar system in which reaction media has many technological and prospective applications. Hence, it seems necessary to understand the properties of reverse micelles. In the diphilic system, both substances with polar and non polar groups may be dissolved and the aggregates of surfactant, act as tiny reactors of molecular size by entrapping enzyme molecules and required reagents. This kind of set up allows controlled local reactions in a limited volume to organize catalytic ensembles of controlled sizes.
There are three major reasons which justify the use of micellar system for fine chemistry:
- Facilitating transformations of water-insoluble substances without diffusion restriction in pseudohomogeneous media.
- By lowering the water content which allows the equilibrium state of catalyzed reactions to be regulated.
- Due to the enzyme incorporation in to the micelles, it allows technological stabilization effects both on the pure state and in combination with other approaches.
Hence, for the chemical manipulation of the proteins, reverse micelles are an excellent and unique tool.
9.1. Protein Hydrophobization
Introduction of hydrophobic and low-water-soluble reagents into protein molecules in controlled quantities for e.g. one or two hydrophobic residues per protein molecule] can be carried out using reverse micelles [32]. In this method, long-chain fatty acid residues [32-34], phospholipids, hormones like thyroid and steroid and metallo-organic compounds such as ferrocene into protein molecules [35, 36] are successfully introduced into the reverse micelles system.
9.2. Construction of Protein Ensembles of Assigned Stoichiometry
As the size of the inner cavity can be manipulated by using different surfactant hydration ratios, it can be made convenient to entrap a larger amount of protein in one micelle [29]. Reverse micelles can also be used to entrap oligomeric forms of proteins due to the variation in the micelle size. In this case, whole set of different oligomeric forms can be obtained: for many individual enzymes and enzyme mixtures. It is also possible to construct nonconventional protein: protein complex using by employing a micellar matrix for example a compact non-covalent chymotrypsin dimer or a stable [dissociating in water only in the presence of 8M urea] non-covalent complex of chymotrypsin with peroxidase [30].
9.3. Generating Protein Complexes with Polymers
Protein molecules are difficult to form in homogeneous solution, but by employing classical linking reagents, it allows the formation of complexes in an “intramicellar” mode. This is one of the methods by which protein complexes, protein conjugates and synthetic polymers of different stoichiometries can be obtained. This process occurs at a low degree of hydration when micelle size is small and after reaching a certain critical degree of surfactant, hydration ratio is adjusted in such a way that a complex is formed which may be linked chemically with a practically quantitative yield. Further increase in micelle sizes may lead to the formation of protein-polymer complexes of higher stoichiometry [34, 37].
9.4. Nanogranulated [Nanocapsulated] Proteins [Enzymes]
It is not always necessary to use existing polymer to prepare nanocapsulated proteins. Reverse micelles can also be used as a medium where monomers undergo polymerization in the micelles and surround the protein molecule. This kind of modification requires chemical modification of enzyme with chemicals like aryl alcohol followed by nanogranulating and nanocapsulating of biologically active compound [37]. Such preparations can be easily recovered by destabilizing the micelles by precipitation with acetone. Such particles contain a covalently entrapped and highly stable enzyme which are having the sizes in the order of tens of nanometers. These particles after dissolving in water or organic solvent can be used as the biocatalyzers. The solubility of such nanoparticles in organic medium may be improved by incorporating hydrophobic fragments into the particles by copolymerization with surfactants containing double bond in the hydrophilic part. Reverse micelles can also be used to obtain nanogranules from various polymers like gelatin or polyvinyl alcoholused for cryogel preparation or formation of organogels based on lecithin [10].
9.5. Enhance the Separation Efficiency of Partition Chromatography
Micellar Electro kinetic Chromatography (MEKC) is based upon the difference in the equilibrium between an aqueous phase and micellar phase. In this technique, due to a combination of electrophoresis & electro osmosis different phases are moving at different velocities. In this technique, the surfactant act as a pseudo-stationary phase and it is utilized above its critical micelle concentration [CMC]. In order to separate both charged and neutral molecules, individually or simultaneously, including chiral compounds, MEKC is a useful technique. Due to Electro- Osmotic Flow (EOF), MEKC benefits from high peak efficiency. MEKC is the method of choice for separation scientists besides a large variety of synthetic surfactants, organic modifiers, temperature and variable separation voltage techniques available for the separation of a variety of compounds [38].
9.6. Shifting an Equilibrium of Various Reactions
Microemulsion-based organogels [MBGs] for the retainment of the catalytic activity was used by Dandvate and Madamwar (2008) in esterification reaction. In order to prevent back hydrolysis by water which is produced as a byproduct in such reaction the removal of water becomes essential so that reaction can proceed in the forward direction. In order to address this fact, pretreatment and/or several intermittent treatments were given to the lipase-containing microemulsion-based organogels with dry reverse micelles AOT based solution in organic solvent during repeated cycles of ester synthesis. The pretreatment of MBGs with dry reverse micellar solution reduced water content and hence higher initial rates of esterification were achieved as compared to untreated freshly prepared MBGs. This treatment has not only improved esterification efficiency but also enhanced stability of the MBGs. Pretreated MBGs have retained 80% esterification efficiency even after the 8th cycle of reaction and extended further up to 9th cycle when pretreatment was again given after the 3rd cycle of esterification. The granulated MBGs showed 1-2 fold increase in the initial esterification rates compared to the pelleted MBGs [39].
9.7. Downstream Processing of Valuable Product
Reverse micelles can be effectively used for the liquid:liquid extraction procedure. However, issues related to widespread use of reverse micelles such as the identification and development of suitable surfactants and ligands; as well as difficulties in the back extraction process have impeded the use of this system [40].
Extracellular acid phosphatase from fermentation broth was extracted by liquid:liquid separation using reverse micelles. AOT: Isooctane based reverse micelles gave maximum extraction of 29% at 25°C at pH 8.0 and with 75% enzyme activity transfer at 50mM KCl using a 3:1 aqueous:organic volume ratio for the forward transfer while on aqueous:organic volume ratio of 1:1 was optimal for the backward transfer [40].
9.8. Bioremediation of Phenolic Environmental Pollutants
Reverse micelles are potential candidates for the treatment of phenolic environmental pollutants like Bisphenol A. Such compounds are insoluble in water therefore organic solvents are essential to dissolve it. In order to degrade them reaction must proceed in organic solvents. Reverse micelles based enzyme preparation exhibit significant catalytic activities in organic media. Enzymes like laccase can oxidize such compounds and subsequently degrade them when entrapped in reverse micelles system, perform biodegradation reaction in an organic solvent and degradation of hydrophobic pollutant is facilitated [41].
CONCLUSION
Reverse micelles are relatively simple but are a valuable tool for the study of various aspects of life science. Reverse micelles are controlled by various factors, like detergent type, detergent concentration, water content, hydration ratio, organic solvent etc. By manipulating these variables of the system, we can explore enzymes and proteins chemistry in a better way and gain useful information about the processes that take place in the physiological systems. Reverse micelles also act as a nanoreactor to perform many unconventional reaction like trans-esterification, separation of valuables and biodegradation of hydrophobic pollutants. More applications using reverse micelles are anticipated in various fields of science in the near future.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


