All published articles of this journal are available on ScienceDirect.
Brown Seaweed Polysaccharide Extraction: Enzyme-Assisted Techniques and Magnetic Nanoparticle-Based Immobilization
Abstract
Brown seaweeds are rich in bioactive polysaccharides such as laminarin, fucoidan, and alginate, which exhibit a wide range of biological activities and hold great potential for applications in the functional food and nutraceutical industries. In recent years, there has been a growing interest in developing advanced and sustainable extraction techniques to improve the recovery of these valuable compounds. While conventional methods – including Soxhlet extraction, hydrodistillation, and maceration – are still commonly used, they are often time-consuming, inefficient, and environmentally taxing. In contrast, innovative techniques such as enzyme-assisted extraction (EAE), microwave-assisted extraction, and ultrasound-assisted extraction offer faster, more selective, and eco-friendly alternatives. Among these, EAE has emerged as a particularly promising approach due to its efficiency, mild operating conditions, and ability to preserve the integrity of thermolabile compounds. However, challenges related to enzyme stability and reusability limit its industrial application. To address these issues, enzyme immobilisation has been explored, with magnetic nanoparticles (MNPs) gaining considerable attention as effective supports due to their large surface area, biocompatibility, and ease of magnetic separation. This review provides an overview of the biology of brown seaweeds and their major bioactive polysaccharides, followed by a critical evaluation of enzyme immobilisation methods. Particular emphasis is placed on the use of MNPs as supports for immobilised enzymes in the context of polysaccharide extraction. The integration of immobilised enzymes with green extraction technologies offers a promising route toward more efficient, sustainable, and scalable recovery of marine-derived bioactives.
1. INTRODUCTION
In recent decades, there has been a growing awareness of the link between diet and overall well-being, resulting in increased consumer demand for food products that deliver health-promoting effects beyond their basic nutritional roles [1]. This trend has driven the global food industry to develop functional foods that cater to health-conscious consumers [2]. In response to growing consumer demand for health-enhancing products, bioactive compounds from natural sources – particularly those derived from plants – have been increasingly explored for applications in the food, cosmetic, and pharmaceutical industries [3]. Within this context, marine algae – especially seaweeds – have attracted significant attention as a rich and sustainable source of bioactive compounds with diverse physiological effects. Their potential aligns closely with the development of functional foods, which are defined as conventional, fortified, or enriched food products that, when consumed regularly in adequate amounts, offer health benefits beyond basic nutritional needs, such as contributing to disease prevention or overall well-being [4].
Seaweeds are macroscopic marine algae that have been part of human history for approximately 14,000 years [5]. These large, non-vascular marine plants are visible to the naked eye and exhibit a wide range of morphologies. According to Guiry & Guiry [6], around 177,142 seaweed species have been identified, varying greatly in shape and size. Seaweeds are commonly classified based on their pigmentation into three major groups: brown, red, and green algae [7, 8]. They are rich in chemical constituents such as carbohydrates, proteins, polyunsaturated fatty acids, minerals, polyphenols, and pigments – many of which exhibit diverse biological activities. Notably, carbohydrates constitute roughly 76% of seaweed biomass, highlighting their potential as a key functional ingredient [9].
Numerous studies have demonstrated the health-promoting effects of seaweed-derived compounds, including antioxidant, anticancer, antidiabetic, antimicrobial, anticoagulant, antiviral, anti-tumor, anti-inflammatory, immunomodulatory, prebiotic, and cholesterol-lowering activities [9]. Despite this, the role of brown seaweeds as prebiotics has received relatively limited attention in the literature, with only a few studies addressing this topic [10-13]. More comprehensive discussions can be found in the reviews by O’Sullivan [14] and de Jesus Raposo et al. [15]. Although in vitro studies have demonstrated the prebiotic potential of brown seaweeds, their broader recognition as prebiotic agents has been hindered by a lack of extensive in vivo evidence, limited human clinical trials, and minimal commercial utilization of brown seaweed-derived polysaccharides [16].
Beyond their health benefits, seaweeds have garnered increasing interest as a sustainable resource due to their rapid growth and minimal input requirements – unlike terrestrial crops, they do not require arable land, fresh water, or synthetic fertilizers [17]. Seaweed farming is now practiced in approximately 50 countries, and in 2014 alone, 28.5 million tons of seaweed and related algae were harvested for direct consumption or as raw material for hydrocolloids, fertilizers, and other industrial products [18]. In Malaysia, seaweed has been recognized as a strategic commodity under the National Agro-Food Policy 2011-2020, with Sabah, located on the eastern coast, serving as the primary center of national production [19, 20].
Given the vast biological potential of seaweed-derived compounds, there has been considerable interest in their extraction and characterization. Various extraction techniques have been employed to obtain bioactive substances from seaweeds for use in food, cosmetic, pharmaceutical, and other applications. Conventional methods such as Soxhlet extraction, hydro-distillation, and maceration have been widely used [21]. More recently, novel and sustainable extraction approaches – such as enzyme-assisted extraction (EAE), microwave-assisted extraction, ultrasound-assisted extraction, supercritical fluid extraction, and accelerated solvent extraction – have been developed. These methods often enhance extraction efficiency while reducing reliance on harsh chemicals and solvents [22]. This mini-review aims to provide an overview of current knowledge on bioactive polysaccharides derived from brown seaweeds, with emphasis on their extraction using enzyme-assisted techniques and the emerging application of magnetic nanoparticle-based enzyme immobilization systems.
2. METHODOLOGY: DATA MINING & SEARCH STRATEGY
The literature search was conducted using Google Scholar and ScienceDirect, applying predefined search terms such as Sargassum, brown seaweed polysaccharides, extraction, immobilization, and nanoparticles. The search was limited to English-language, peer-reviewed original and review articles published between 1975 and 2025, to capture both early foundational studies and more recent advances. Relevant articles were then selected based on their scope and quality for inclusion in this review.
3. MARINE MACROALGAE
The term ‘algae’ refers to a diverse group of polyphyletic, mostly photosynthetic organisms with diverse origins, evolutionary lines, and biochemistry. Algae taxonomy is presently undergoing reassessment and enhancement through the application of molecular genetics. Practically, algae can be categorized into two groups: multicellular marine organisms (macrophytes and seaweeds) and unicellular or colonial microalgae that can be found in many habitats such as seas, freshwater lakes, rivers, ponds, and soil [23]. Seaweeds are macroalgae found in maritime environments and have a significant fossil record [5]. Seaweeds are typically categorized based on their chemical and morphological characteristics, particularly their pigmentation, which determines their classification into one of three algal divisions: brown, red, and green algae. Brown algae, also known as Phaeophyceae, are the largest type of algae. It can appear brown or yellow-brown owing to the presence of the compound fucoxanthin. On the other hand, red algae, or Rhodophyceae, display vibrant colors because of the dominance of phycoerythrin and phycocyanin over other pigments such as chlorophyll a, β-carotene, and various xanthophylls. Lastly, green algae, or Chlorophyceae, possess chlorophyll a and b in the same proportion as higher plants [7, 24].
3.1. Brown Seaweeds
Brown seaweeds are classified phylogenetically as Chromista and Ochrophyta [6]. Brown seaweeds and higher plants possess similar interesting characteristics, such as totipotent cells and intercellular plasmodesmatic connections, which play a vital role in the development of multicellular organisms. Of the three primary categories of seaweeds, the brown seaweed appears to be the most influenced by climatic conditions, resulting in variations in its characteristics depending on the geographical location [25].
Brown seaweeds possess a gelatinous cell wall that consists of two layers: an inner layer predominantly made up of cellulose and an outer layer primarily formed of algin and fucoidan. In addition, they contain amorphous mucilaginous matrix fraction and mucilaginous alginates [25]. Brown seaweeds possess phlorotannins, which are halogenated and sulphated phenolic compounds found in their cell walls [26]. The variations in cell wall composition are not only noticeable between different species and seasons, but even within the same thallus. The variations in cell wall composition are not only noticeable between different species and seasons, but even within the same thallus. Brown seaweeds have a particularly large and complex thallus, which is categorised into many growth types: diffuse, apical, trichothallic, promeristem, intercalary, and meristoderm [27]. Fucoxanthin, a carotenoid pigment, along with other compounds such as Phaeophyceae tannins, β-carotene, chlorophyll a, c1, and c2, diatoxanthin, violaxanthin, and substantial amounts of fucoxanthin, contribute to the brown coloration of brown seaweeds [25].
4. BIOACTIVE POLYSACCHARIDES OF BROWN SEAWEEDS
Brown seaweeds consist of three primary polysaccharides: laminarin, fucoidan, and alginate. Laminarin (Fig. 1), also known as laminaran, is a type of water-soluble polysaccharide [28]. It has a relatively small molecular weight of around 5 kilodaltons. Laminarin is made up of β-(1,3)-linked glucans with β-(1,6)-linked side chains. These side chains have different distributions and lengths, often consisting of about 20 to 25 glucose units [29]. These compounds are located in the vacuoles of cells, make up around 35% of the dry weight of brown seaweed, and serve as storage polysaccharides [14, 22]. The seaweed species that mostly contain laminarin are Laminaria, Saccharina, Ascophyllum, and Fucus [30]. Studies have shown that laminarin possesses antibacterial, immunomodulatory, antioxidant, and anticoagulant activities [22, 31].
Fucoidans (Fig. 2), sometimes referred to as fucan or fucosan, are a group of sulphated homo- and heteropolysaccharides mostly made up of α-(1,2)- and/or (1,3)-linked fucose [32, 33]. These compounds can dissolve in water and diluted acids, and their molecular weight ranges from 100 to 1600 kDa [31]. Fucoidans are abundant in brown seaweed fibrillar cell walls and intercellular spaces. The substitution of sulphates most commonly occurs at the C2 and C4 locations of L-fucopyranosyl residues, but not at the C3 position [34]. Fucoidan extracts not only consist of fucose, but also galactose, mannose, xylose, glucose, and glucuronic acid [34]. Fucoidans are predominantly present in the Fucaceae and Laminariaceae groups of algae, which are found globally [35]. Fucoidans have demonstrated a broad spectrum of bioactivity, including antiviral, anti-inflammatory, immunomodulatory, antithrombotic, anticoagulant, antioxidant, antitumor, antibacterial, and anticancer properties [31, 34, 36, 37].
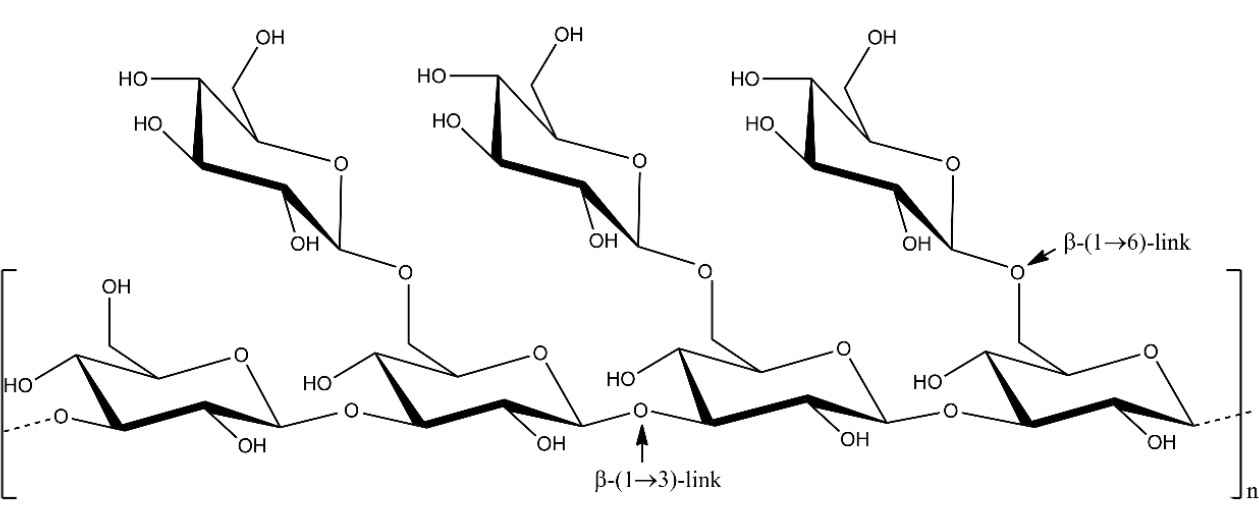
Schematic depiction of the structure of laminarin. Source: Redrawn from the work of Kim et al. [28] using ChemDraw.
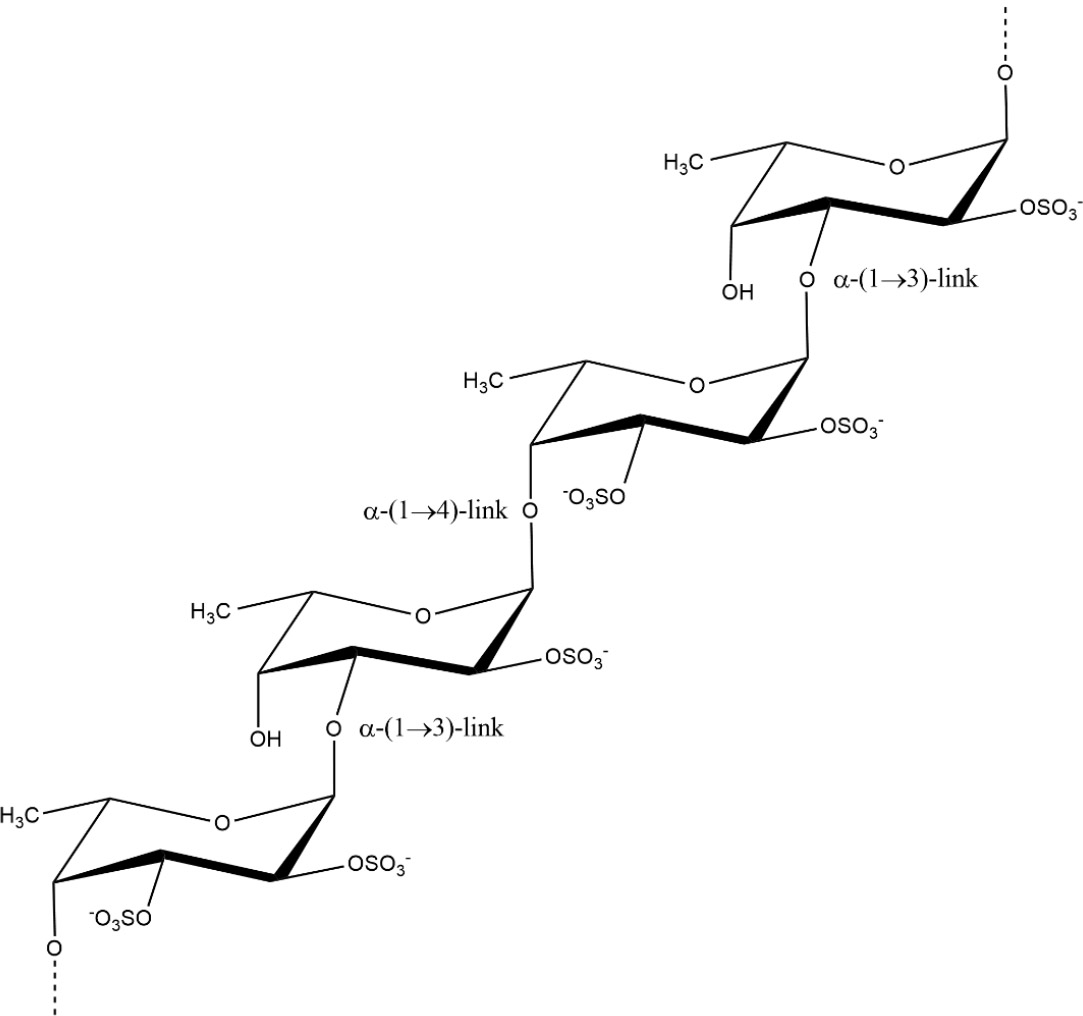
Fucoidan structure from Fucus vesiculosus. Source: Redrawn from the work of Peipei et al. [33] using ChemDraw.
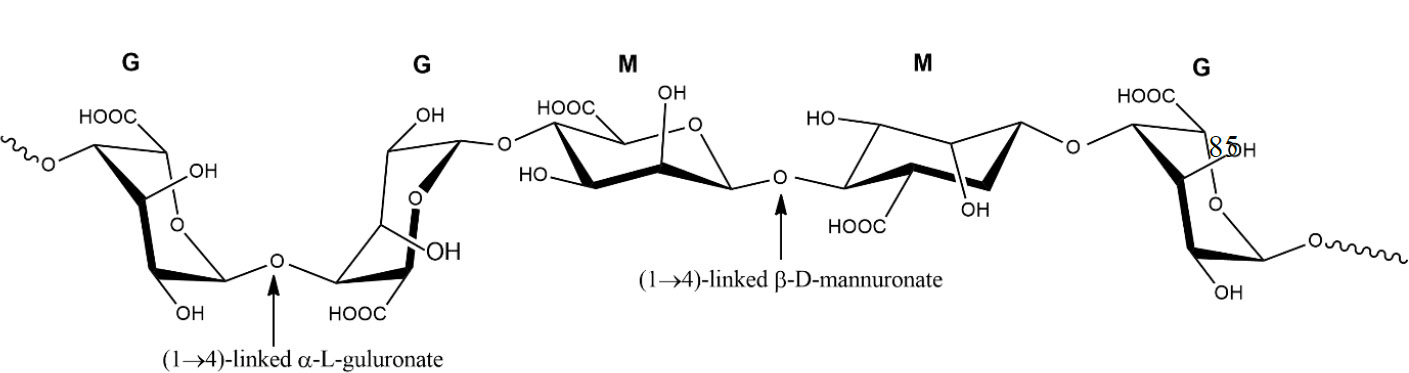
Alginates (Fig. 3) are unbranched homopolymers composed of β-D-mannuronic acid (M) and α-L-guluronic acid (G). There are three distinct types of alginate blocks: poly-M, poly-G, and alternating MG [38, 39]. Every type has a unique arrangement of mannuronic acid and guluronic acid in the alginate chain [40]. The chemical composition and monomeric sequence of the extracted alginate differ depending on the specific algae species, the portion of the algae employed, the season, and the prevailing oceanic conditions [41]. Alginates are plentiful in the cell walls of brown seaweed [14]. Marine alginate is obtained from many types of seaweed, including Phaeophyceae, Laminaria, Ecklonia, Ascophyllum, Durvillaea, Lessonia, Macrocystis, Sargassum, and Turbinaria [42]. They exhibit a well-established gelling property in the presence of multivalent cations or when the pH falls within the range of 3 to 4, and the occurrence of gel formation is related to a significant concentration of guluronic acid [43].
5. EXTRACTION OF BIOACTIVE COMPOUNDS FROM SEAWEEDS
5.1. Pre-treatment
Seaweed is typically harvested along the coast or on beaches. The seaweed undergoes a rigorous washing process to eliminate any salt, impurities, or epiphytes. Before extraction, seaweeds undergo a process of drying and milling to achieve uniform distribution of mass and a greater surface-to-volume ratio [44]. Moreover, these pre-treatments are crucial to prevent the co-extraction of other bioactive compounds from seaweeds that have similar solubility properties [45].
5.2. Conventional Extraction Techniques
Soxhlet extraction, hydro-distillation, and alcohol maceration are conventional techniques used to extract bioactive compounds [46]. Conventional extraction methods are inefficient, time-consuming, energy-intensive, and environmentally unfriendly. Furthermore, these extraction methods are usually performed manually and pose challenges in terms of reproducibility.
5.3. Innovative Extraction Techniques
The increasing demand for marine bioactive chemicals has led to the rapid development of new extraction procedures that are fast, specific, economical, productive, and eco-friendly. Numerous novel extraction technologies have the potential to meet many the requirements outlined above. Enzyme-assisted extraction, microwave-assisted extraction, ultrasound-assisted extraction, supercritical fluid extraction, and accelerated solvent extraction are all exemplifications of these techniques [47]. The objective of this review is to thoroughly examine a novel and nontoxic method termed enzyme-assisted extraction, as well as the immobilisation of enzymes for extraction.
5.4. Enzyme-assisted Extraction
The composition of the cell wall in seaweed is quite complex, consisting of a diverse range of polymeric biomolecules. These include sulphate and branched polysaccharides, as well as proteins and various bound ions like calcium and potassium [48, 49]. The complex structure of seaweed cell walls, along with their sturdy nature, presents significant challenges when it comes to efficiently extracting bioactive compounds. Enzyme-assisted extraction (EAE) operates under the principle of utilizing enzymes to break down the cell wall of seaweed, thereby simplifying the process of extracting desired components from seaweed materials. Research has demonstrated that certain enzymes, such as carbohydrases and proteases, can effectively break down the cell wall of seaweed and release specific bioactive compounds. This process occurs under specific temperature and pH conditions, as highlighted in a study by Kadam et al. [22].
EAE is considered to be a safe and eco-friendly method as it eliminates the need for solvents in the extraction process. This technology is highly efficient in breaking down cell walls and releasing valuable bioactive compounds. In addition, it addresses challenges related to the water solubility and insolubility of biologically active compounds. This technology is a cost-effective method that makes use of enzymes that are commonly present in food. These enzymes include cellulase, α-amylase, and pepsin [50]. EAE offers the benefits of high catalytic efficiency while maintaining the original effectiveness of the compounds to a considerable extent [51]. For optimal extraction yield, protocols in the field emphasize the significance of maintaining optimal treatment time and temperature conditions for enzymes [50].
Although EAE offers several benefits, it also comes with certain drawbacks. These include being sensitive to changes in pH and temperature, having limited stability, and being challenging to recover and reuse in a reaction system. As a consequence, the use of EAE can lead to high operating costs. Various techniques are employed to enhance enzyme stability, such as immobilization, enzymatic modification, and protein engineering. One widely used technique for enhancing enzyme properties involves immobilization [52, 53].
Figure 4 presents a schematic representation of the extraction stages for bioactive compounds from seaweeds, encompassing conventional and innovative techniques with their respective advantages and limitations.
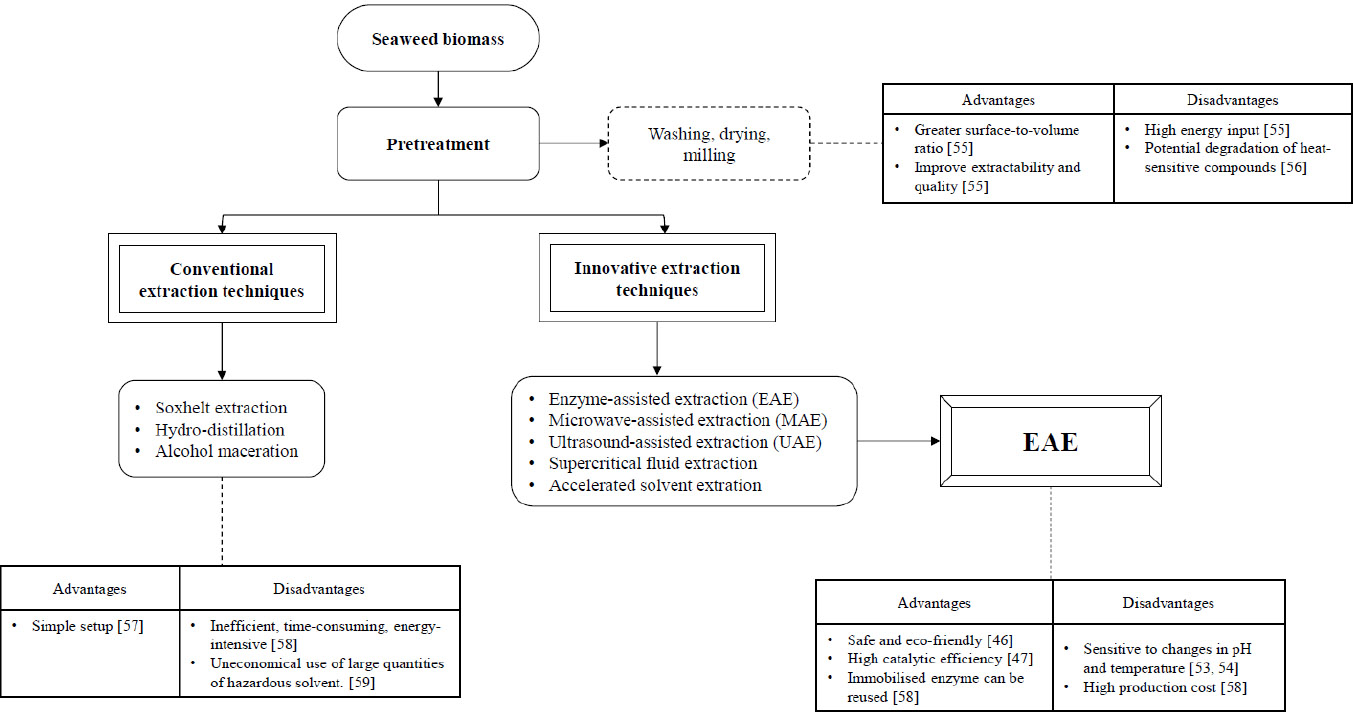
Schematic representation of bioactive compound extraction from seaweed, highlighting pre-treatment, conventional and innovative methods, with emphasis on EAE and associated trade-offs.
6. ENZYME IMMOBILISATION TECHNIQUES
Enzyme immobilisation aims to keep the enzyme securely bound and confined within a physical medium, thereby preserving its catalytic activity [53]. The immobilization of enzymes offers several benefits. One advantage is the convenience of handling the enzyme as a solid rather than a liquid, which allows for easy separation from the product and reduces or eliminates protein [54]. In a recent study by Kalsoom et al. [55], it was found that enzymes immobilized on nanoparticles exhibited a broader range of pH and temperature tolerance, along with enhanced thermal stability compared to their free form. According to Cao [56], the fundamental characteristics of immobilized enzymes are their simplicity, profitability, and stability, which are highly valued in the industrial sector. Figure 5 provides an overview of the various immobilization methods currently in use.
Enzymes can be immobilized using two main methods: physical and chemical. In the physical methods, enzymes are immobilized onto different matrices by the use of forces such as van der Waals forces, hydrophobic interactions, and hydrogen bonding. Adsorption onto a support surface is the simplest form of immobilization. The enzyme usually binds to the support material through non-covalent linkages, such as ionic and hydrophobic interactions, hydrogen bonding, and van der Waals forces, without requiring any prior activation of the support [57]. The adsorption method is straightforward and does not require complex chemical reactions, making it economically viable for large-scale applications [58, 59]. While enzyme immobilization by adsorption offers several advantages, such as simplicity and cost-effectiveness, it is important to consider the potential drawbacks, including enzyme leaching [60] and reduced activity [61].
Entrapment involves the confinement of the enzyme within a polymer matrix, such as gel or a membrane [58]. This allows for the penetration of substrates and products while restricting the release of enzyme proteins [62]. Consequently, the application is restricted to processes that only involve small substrates and products. Some of the techniques employed include entrapment within gels like polyacrylamide and calcium alginate, as well as entrapment within hollow fibres or the microcavities of synthetic fibres [62, 63]. Entrapment of the enzyme does not involve any chemical modification, thereby resulting in minimal alteration of the enzyme's properties. The method has several constraints, such as enzyme leakage, restrictions in mass transfer/diffusion due to the movement of substrates and products via a barrier, and the absence of stabilizing effects typically found in a solid support [62, 64].
Microencapsulation is a technique used to immobilize enzymes by encapsulating them within spherical, semi-permeable polymer membranes with controlled porosity typically ranging from 1 to 100 μm [65]. These membranes permit the selective transport of molecules, allowing substrates to diffuse in and reaction products to diffuse out while retaining the enzyme within the capsule [66].
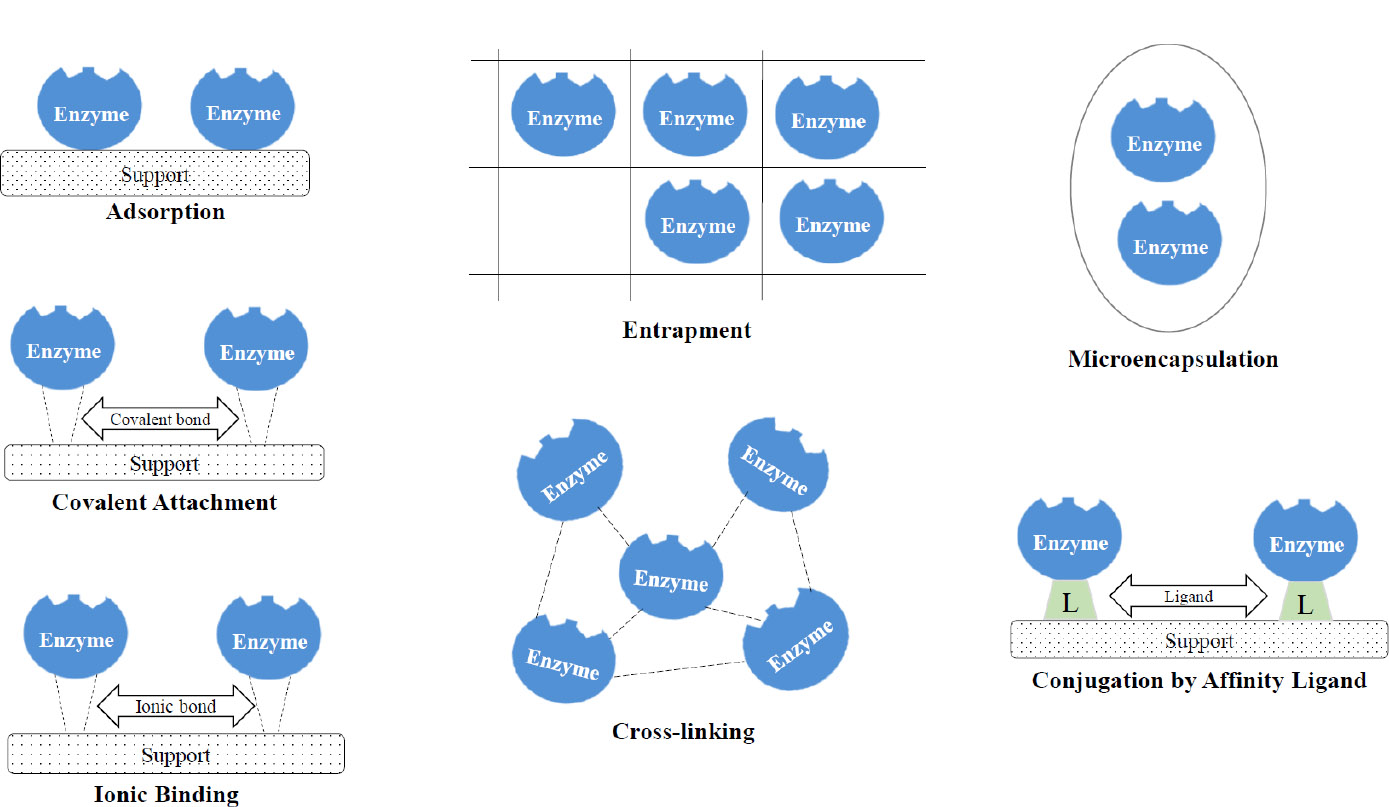
Overview of enzyme immobilization methods.
This selective permeability creates a controlled microenvironment that enhances enzyme stability and catalytic activity by protecting the enzyme from proteolytic degradation and harsh external conditions [66-68]. Furthermore, the encapsulation matrix can modulate the release of enzymes, thereby optimize substrate-enzyme interaction and improving overall reaction efficiency [69]. Despite these advantages, microencapsulation also presents certain limitations. Enzymes may desorb from the membrane surface, leading to diminished catalytic availability [64]. Additionally, the mechanical integrity of the semi-permeable membranes can be compromised under operational stresses, resulting in rupture or degradation that may cause enzyme leakage and a consequent loss of enzymatic activity [70].
In the chemical method, enzymes are permanently attached to different matrices through covalent or ionic bonds [71]. Covalent binding is considered the most secure approach for immobilizing enzymes. Enzyme molecules can be attached to reactive groups on the carrier, such as hydroxyl, amide, amino, or carboxyl groups [71]. Alternatively, a spacer arm can be artificially attached to the carrier through different chemical reactions, like diazotization, Schiff base, or imine bond formation. Non-essential amino acid residues (other than active site groups) of the enzyme participate in the formation of a covalent bond, resulting in minimal conformational alterations. It is worth noting that immobilized enzymes via covalent and ionic bonding exhibit greater resistance to various physical and chemical conditions, such as temperature fluctuations, denaturants, and organic solvents [72, 73]. Nevertheless, due to the rigorous immobilization conditions and the existence of identical amino groups at the enzyme's active site while in contact with the matrix, this particular method of immobilization places an increased strain on the enzyme and occasionally leads to significant alterations in both its conformational and catalytic properties [73].
Cross-linking is an immobilization technique that involves the formation of covalent bonds between enzyme molecules and a supporting matrix using bi- or multifunctional reagents, resulting in the creation of a three-dimensional network known as cross-linked enzyme aggregates (CLEAs) [74]. This process is commonly facilitated by bifunctional agents such as glutaraldehyde, which reacts with amino groups on the enzyme surface to form stable linkages [59, 75]. Glutaraldehyde oligomers, formed via aldol condensation, can further enhance enzyme activity by forming additional cross-links, thereby improving the structural integrity and catalytic performance of the immobilized enzyme [75]. The primary advantages of CLEA technology include its operational simplicity, enhanced enzyme reusability, and improved thermal and chemical stability [60, 74, 76]. However, certain limitations remain. The use of harsh cross-linking conditions or excessive reagent concentrations can induce enzyme denaturation, adversely affecting catalytic activity and substrate specificity [77]. Moreover, the dense structure of CLEAs may result in internal mass transfer limitations, thereby reducing the overall reaction efficiency. Addressing these challenges is essential for optimizing the practical application of CLEAs in biocatalytic processes [77].
Ionic binding involves the electrostatic interaction between charged enzyme molecules and oppositely charged support matrices. This approach is facilitated by various materials, including ionic liquids (ILs), which can function as additives, linkers, or surface modifiers. ILs play a critical role in modulating enzyme-support interactions, thereby influencing enzyme stability, activity, and overall immobilization efficiency [78, 79]. Inorganic materials such as silica are frequently employed due to their high surface area, mechanical strength, and compatibility with both ionic and covalent immobilisation strategies [80]. Similarly, synthetic polymers like polyamides offer a conducive environment for enzyme immobilisation via ionic interactions, supporting enzyme stability and activity [71]. Enzymes immobilized through ionic binding can be readily separated from reaction mixtures, facilitating their reuse in continuous or batch processing systems. This recyclability contributes to reduced enzyme consumption and lowers operational costs in industrial applications [70, 81]. However, ionic interactions can sometimes induce conformational changes in the enzyme, particularly around the active site, potentially diminishing catalytic performance [79]. Furthermore, the binding process may compromise enzyme structural integrity over time, leading to a gradual loss of functionality [58]. In addition, not all enzymes interact favorably with ionic supports, which can limit the broad applicability of this immobilization technique [70].
Affinity immobilisation offers an alternative strategy for enzyme immobilisation by exploiting specific interactions between the enzyme and an affinity ligand. Common systems include biotin–avidin and metal chelate–histidine tag interactions, which enable strong and selective binding of the enzyme to the support matrix [82, 83]. These highly specific interactions minimise the risk of random immobilisation and reduce the likelihood of active site obstruction, a common drawback in conventional immobilisation techniques [83]. This method is particularly advantageous in applications requiring high sensitivity and specificity, such as biosensor development, where the stability and functional integrity of the immobilised enzyme are essential for accurate detection [84]. The strong affinity bonds formed during immobilisation enhance enzyme stability, prevent leaching, and support reusability over multiple operational cycles. Furthermore, affinity immobilisation allows for oriented enzyme attachment, preserving catalytic activity and improving performance in both analytical and industrial applications [82]. However, the widespread adoption of this technique is limited by challenges such as the high cost of affinity ligands and the potential for steric hindrance, which may affect enzyme conformation and activity [85]. Addressing these limitations is crucial to expanding the applicability of affinity-based immobilisation systems.
7. MAGNETIC NANO-SUPPORTS FOR ENZYME IMMOBILIZATION
Nanoparticles are defined as discrete entities with dimensions of 100 nm or less in all three spatial directions. Their unique physicochemical properties, distinct from those of bulk materials, have enabled their broad application across various fields [86]. Among these, magnetic nanoparticles (MNPs) have emerged as promising platforms for enzyme immobilisation, offering a combination of features that enhance enzyme activity, stability, and reusability.
MNPs are typically composed of magnetic elements such as iron, cobalt, and nickel, or their corresponding oxides and compounds. In particular, iron oxide nanoparticles – magnetite (Fe3O4) and maghemite (γ-Fe2O3) – have been widely utilised due to their biocompatibility and superparamagnetic behaviour [87]. Enzyme immobilisation on MNPs can be achieved through various methods, including physical adsorption, encapsulation, covalent bonding, and cross-linking. Physical adsorption is a simple, reversible technique relying on non-covalent interactions, whereas encapsulation involves trapping enzymes within a polymer matrix on the nanoparticle surface, offering structural protection and stability [88, 89]. Covalent immobilisation, on the other hand, forms stable linkages between enzyme functional groups and activated surfaces of MNPs, ensuring strong attachment and reduced leaching [90, 91]. Cross-linking techniques, often employing agents such as glutaraldehyde, can create networks of enzyme aggregates on the nanoparticle surface, further enhancing stability and reusability [87, 92].
The high surface-area-to-volume ratio of MNPs facilitates increased enzyme loading, thereby improving catalytic efficiency [93]. Surface modification strategies – such as silanization with 3-aminopropyl-triethoxysilane (APTES) – enhance enzyme binding, maintain activity across broad pH and temperature ranges, and improve operational robustness [87, 93]. As a result, enzymes immobilised on MNPs often exhibit enhanced thermal and operational stability, extended functional lifespans, and reduced degradation over repeated use cycles, making them well-suited for industrial bioprocesses [94, 95].
A major advantage of MNP-based systems lies in their superparamagnetic nature, which allows for rapid and efficient separation of immobilised enzymes from reaction mixtures using an external magnetic field. This simplifies the recovery and recycling process, reducing operational costs and contamination risks [96]. To further improve biocompatibility and prevent oxidation, MNPs can be functionalised with protective coatings through methods such as silanization or carbodiimide activation, which enhance stability and enzyme retention [93]. Some MNPs, such as nickel ferrite, have demonstrated the ability to immobilise enzymes directly from crude cell lysates, streamlining the immobilisation process and reducing the need for extensive purification or chemical treatments [97]. However, despite their advantages, several limitations remain. Uncoated MNPs are susceptible to oxidation, which may compromise both nanoparticle integrity and enzyme activity, thus necessitating protective surface modifications [98]. Additionally, diffusion limitations within densely packed systems can hinder substrate access and reduce catalytic efficiency [99]. Moreover, the need for precise surface functionalisation to improve enzyme binding and biocompatibility can complicate the design and synthesis of immobilised systems [93, 100].
8. BIOACTIVE RECOVERY VIA IMMOBILISED ENZYMES
The advancement of enzyme technology has markedly improved the extraction of bioactive compounds from seaweeds, which are abundant in antioxidants, polysaccharides, and other high-value metabolites. Among these innovations, the application of immobilised enzymes in enzyme-assisted extraction (EAE) has gained considerable attention. Incorporating immobilised enzymes into EAE systems enhances both the yield and specificity of target compound recovery, while also improving enzyme stability, extending catalytic lifespan, and enabling repeated use [101-104]. These benefits are largely attributed to immobilisation techniques such as covalent binding, entrapment, and adsorption onto solid supports, which contribute to greater process efficiency and sustainability in industrial-scale applications [105, 106].
In addition to enhancing yield and selectivity, immobilised enzymes exhibit excellent tolerance to harsh processing conditions, including elevated temperatures and broad pH ranges, making them robust biocatalysts for diverse extraction environments [107, 108]. Their reusability simplifies downstream processing by facilitating enzyme recovery and reducing contamination risks, ultimately lowering operational costs [109]. Such operational advantages are critical in large-scale production systems where process reliability and cost-efficiency are essential.
Empirical studies have validated the effectiveness of immobilised enzymes in extracting bioactives from brown seaweeds. Hydrolytic treatments of brown algae have significantly increased the release of compounds such as phlorotannins and sulfated polysaccharides, known for their antiviral and anti-inflammatory properties [110]. Compared to free enzymes, immobilised systems consistently yield higher concentrations of desired metabolites [111]. For example, laccase from Trametes versicolor, immobilised via cross-linked enzyme aggregates, demonstrated improved pH stability, and maintained high catalytic efficiency across multiple cycles in the degradation of bisphenol A – a proxy for persistent organic pollutants – highlighting the broader applicability of immobilisation for extraction purposes [108].
To overcome the limitations associated with free enzyme systems, such as rapid inactivation and recovery challenges, magnetic nanoparticles (MNPs) have been employed as enzyme carriers. MNPs offer high surface area and tunable surface chemistry, enabling improved enzyme loading, enhanced catalytic activity, and facile magnetic recovery, thus streamlining downstream processing and supporting enzyme reusability [112].
Beyond seaweeds, immobilised enzymes have also been successfully applied in the selective extraction of pharmacologically active compounds from complex plant matrices. Ligand fishing techniques, which utilise immobilised enzymes on solid supports, allow for the targeted isolation of bioactive molecules such as flavonoids and polyphenols through affinity interactions, offering valuable insights into their therapeutic potential [113]. Similarly, in citrus peel extraction, immobilised pectinases have facilitated cell wall degradation, enhancing the release and solubilisation of antioxidants and flavonoids [114]. These examples underscore the versatility and applicability of enzyme immobilisation across diverse biological sources.
To further optimise extraction performance, recent studies have explored co-immobilisation of multiple enzymes. This approach promotes substrate channelling, minimises mass transfer limitations, and enhances catalytic synergy, leading to improved enzymatic activity and overall process stability [115]. Additionally, integrating immobilised enzymes with advanced extraction techniques – such as ultrasound-assisted extraction and supercritical fluid extraction – has been shown to improve extraction efficiency while preserving the structural integrity and bioactivity of the compounds of interest [116]. The adaptability of enzyme immobilisation also enables tailoring of catalytic systems to specific substrates and product profiles. The use of novel support materials, such as mesoporous silica, has proven effective in optimising enzyme loading, distribution, and kinetic performance during extraction processes [117]. This level of customisation broadens the potential applications of immobilised enzyme systems for extracting bioactives from a wide range of marine and terrestrial biomass.
Figure 6 shows the schematic workflow for enzyme immobilisation onto magnetic nanoparticles (MNPs) for bioactive compound extraction. The process begins with the synthesis of MNPs, followed by surface modification or functionalisation (e.g., –NH2 or –COOH groups) to facilitate enzyme binding. Enzymes are then immobilised onto the modified MNPs, purified, and characterised. The immobilised enzymes are applied for bioactive extraction and recovered via magnetic separation. The cycle continues with either reuse or final disposal/application, depending on enzyme stability and performance.
In conclusion, enzyme immobilisation represents a powerful advancement in bioactive compound extraction. It offers enhanced catalytic stability, reusability, and selectivity, while facilitating cost-effective and sustainable processing. These advantages position immobilised enzyme-assisted extraction as a highly promising strategy for the nutraceutical, pharmaceutical, and functional food industries, enabling more efficient utilisation of natural resources for value-added product development.
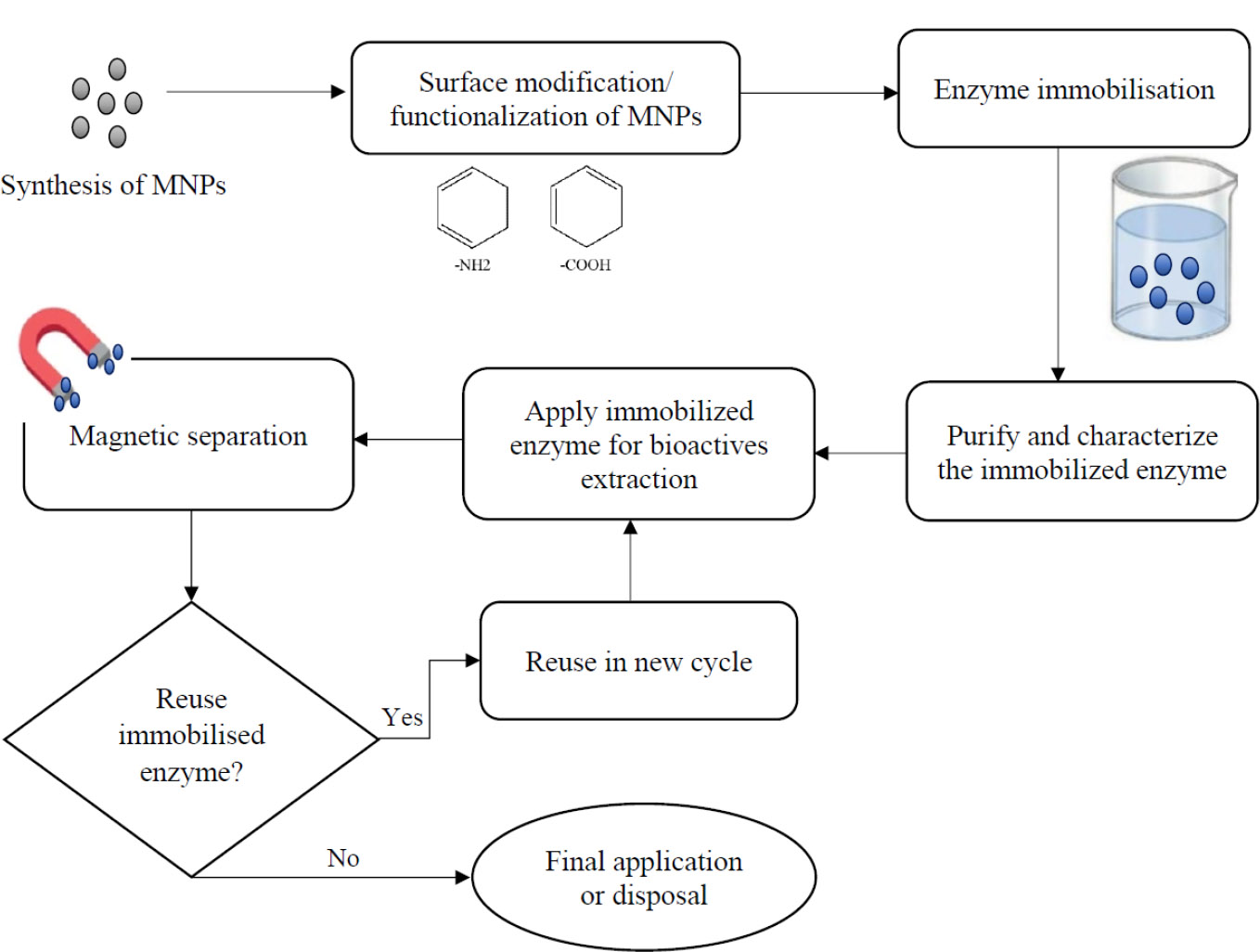
Schematic diagram illustrating the steps involved in enzyme immobilisation onto magnetic nanoparticles for bioactive extraction and reuse.
CONCLUSION
Seaweed is an abundant source of bioactive compounds, particularly polysaccharides. The growing popularity of advanced enzyme-assisted extraction technology has become important for effectively isolating valuable polysaccharides from seaweed. Through the immobilization of enzymes, this technology has the potential to enhance cost-effectiveness. Using magnetic nanoparticles for enzyme immobilization offers several benefits. These include the ease of separating the enzymes, improved stability and activity, higher enzyme loading, and the possibility of using them for multiple functions. The advantages of magnetic nanoparticles make them a highly effective and economical option for biotechnological applications, especially in industrial processes that demand optimal and affordable enzyme utilization while also being environmentally sustainable.
AUTHORS' CONTRIBUTIONS
The authors confirm contribution to the paper as follows: C.C.K: Conceptualization, data curation, formal analysis, investigation, visualization, writing – original draft, writing – review & editing; S.L.H: Conceptualization, funding acquisition, investigation, validation, writing – review & editing; T.S.Y.M: Conceptualization, investigation, writing – review & editing; K.K.W: Conceptualization, investigation, writing – review & editing. All the authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| APTES | = 3-aminopropyl-triethoxysilane |
| CLEAs | = Cross-linked enzyme aggregates |
| EAE | = Enzyme-assisted extraction |
| Fe3O4 | = Magnetite |
| γ-Fe2O3 | = Maghemite |
| G | = α-L-guluronic acid |
| ILs | = Ionic liquids |
| kDa | = Kilodalton |
| M | = β-D-mannuronic acid |
| MNPs | = Magnetic nanoparticles |
| μm | = Micrometer |
| nm | = Nanometer |
| –COOH | = Carboxyl group |
| –NH2 | = Amine group |
FUNDING
This paper has been funded by the Ministry of Higher Education, Malaysia (FRGS/1/2019/WAB09/KUTS/02/1) and the University of Technology Sarawak, Malaysia (UTS/RESEARCH/3/2022/12) supported the study.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Ministry of Higher Education, Malaysia and the University of Technology Sarawak, Malaysia for research funding and technical support. Additionally, the authors acknowledge the use of generative AI tools, specifically QuillBot, to improve the clarity and coherence of the English language in this article.