All published articles of this journal are available on ScienceDirect.
Detection and Mineralization of Organic Pollutants in Mesopotamian Marsh Water Using a Batch Bioreactor
Abstract
Introduction
This study investigated the detection and mineralization of organic pollutants in the Mesopotamian marshes.
Methods
Over the course of seven days of treatment, a batch bioreactor system and natural microbial communities from marsh sediments were able to break down contaminants in a remarkable way.
Results
Water samples collected from five distinct marsh locations revealed significant contamination, with the Central marshes showing the highest pollutant concentrations (aliphatic hydrocarbons, 100 μg/L; phenols, 40 μg/L; aromatic hydrocarbons, 5000 μg/L; and pesticides, 120 μg/L). Notable findings included the near-complete elimination of aliphatic hydrocarbons (85%-99% reduction) across all sites, as well as substantial decreases in aromatic hydrocarbons (79%-92%). Phylogenetic analysis revealed that previously unidentified bacterial species labeled as FF-A5 and FF-A6 were the dominant bacterial species in the batch reactor. These bacterial species have most likely been native bacteria with an exceptional capacity for breaking down pollution.
Discussion
The results suggested the possibility of using native microbial community bioremediation methods to effectively restore marsh ecosystems and lower organic pollution in these important wetlands in a sustained manner.
Conclusion
Customized treatments guided by the synergistic interactions among the several bacterial species revealed in this work could help to improve pollution degradation and ecological restoration of the Mesopotamian wetlands.
1. INTRODUCTION
Among the most extensive and ecologically important wetland settings in the Middle East, the Mesopotamian marshes are also a UNESCO world heritage site [1]. Apart from providing habitats for a great range of species and humans, these wetlands also help to store carbon and clean water [2]. However, during the past few decades, human activity in these wetlands has been under more and more strain, which has resulted in increased pollution levels. Harmful to both aquatic life and human health, organic compounds, including pesticides, phenols, and hydrocarbons, have been found in marsh waters [3, 4].
In the Mesopotamian marshes, human activities have historically led to significant ecological transformations [5]. Consequences of wetland draining brought about by agricultural and urban expansion are the loss of habitat and rising salt levels in the surviving bodies of water [6]. Recent studies [7] indicate that the wetlands are not functioning as a pollution sink. This modification demands quick research on cleanup strategies capable of restoring these important ecosystems to their former state [8].
The main sources of organic contaminants winding into the Mesopotamian wetlands are agricultural runoff, industrial discharge, and oil exploitation. Research [9] suggests that these pollutants could alter ecological equilibrium, taint food webs, and impede the natural cleansing systems of wetlands. A comprehensive knowledge of the sources, distribution, and fate of organic pollutants in the Mesopotamian wetlands is necessary to develop sustainable management techniques aiming at restoring ecological health.
Particularly in relation to organic pollutants and microorganisms, bioremediation has been much discussed as a practical and effective method for environmental cleanup [4]. Organic pollutants, including pesticides, hydrocarbons, and persistent organic pollutants, present major hazards to ecosystems and human health because of their toxicity and incapacity for breakdown [10, 11].
In bioremediation, bacteria are essential since they absorb these contaminants as carbon and energy sources, and then, via metabolic pathways, they transform them into less harmful compounds [12]. Some bacteria, including Pseudomonas, Bacillus, Sphingomonas, and Rhodococcus [13], may biodegrade many organic pollutants under aerobic conditions. Just two of the complicated chemical molecules these bacteria may break down are hydrocarbons and phenolic chemicals. Particularly well-known for effectively breaking down toxic compounds, such as polycyclic aromatic hydrocarbons [13], are species of Pseudomonas. Using bacterial consortia helps to make bioremediation more effective. Cooperative interactions among several bacterial species can lead to better rates of degradation and increased substrate use [14]. By including microorganisms with enhanced pollution breakdown capacity, a technique known as bioaugmentation, which involves adding specific bacterial strains to contaminated areas, may help to improve bioremediation results [15].
Largely due to molecular techniques, especially 16S rRNA gene sequencing, the dynamics of microbial communities participating in bioremediation have become more well-known. By using these techniques, the most prevalent bacterial communities, as well as their roles in pollution degradation, can be revealed [13]. These discoveries are essential to maximizing bioremediation processes that are fit for specific contaminants and environmental conditions. In this study, the bioremediation potential of the Mesopotamian marshes, an ecosystem of global ecological significance that has remained largely uninvestigated, was examined. Indigenous microbial communities present in these waters were employed, as they are naturally adapted to local pollutants. By using native consortia, ecological balance was preserved, and the risks linked to the introduction of non-native organisms were minimized. Through this approach, the important role of native microbes in restoring heavily impacted aquatic environments has been demonstrated in a sustainable and environmentally responsible manner.
This study has thus explored the presence and potential consequences of organic pollutants in the water of the Mesopotamian marshes by means of modern analytical techniques, including gas chromatography-mass spectrometry (GC-MS), to ascertain the degrees and types of contamination in several marsh areas. By means of sustainable management strategies, ecological equilibrium in these wetlands can be restored. Moreover, this study has investigated bioremediation methods that can effectively remove organic pollutants by using natural microbial populations. This work has identified significant pollutants and their spatial distribution to support ecological restoration and assist the long-term preservation of this essential ecosystem. The results of this study can aid in the development of focused therapies. The novelty of this work is that it involved the use of indigenous microbial communities from the underexplored Mesopotamian marsh regions of Iraq for the biodegradation of multiple organic pollutants, along with the identification of uncharacterized bacterial strains, which can likely play a central role in pollutant degradation.
2. MATERIALS AND METHODS
2.1. Study Design
This study employed an experimental analytical approach using a batch bioreactor system to detect and mineralize organic pollutants in water from Mesopotamian wetlands. Researchers set out to ascertain in a controlled laboratory environment as to how efficiently wild microbial communities can break down hydrocarbon contaminants. Molecular techniques and quantitative usage of chemical analysis were employed. Five especially contaminated marsh sites, including Central, Hammar, Hawizeh, and Hammar marshes, had water and sediment samples obtained from them. Mixing a nutrient solution with the homogenized silt produced the batch bioreactor; this mixture provided the microenvironment for the bacteria. Regulated in batch mode under controlled conditions of aeration, temperature (25–30°C), and pH (6.8–7.2), the system declined most effectively. To determine the amounts of organic pollutants, samples were collected every 24 hours and subjected to GC-MS analysis. The dynamics of the microbial community were evaluated using 16S rRNA gene sequencing both before and after the treatment. The results of this experiment have provided strong evidence that biotechnological intervention is the key to mineralization and pollution detection.
2.1.1. . Inclusion Criteria
This study used soil and water samples from five very polluted sites in the Mesopotamian marshes. These sites were chosen because they were sensitive to pollutants from cities, farms, and factories. Analyzed samples were taken throughout the 2024 dry season to confirm environmental stability. Organic contaminants, particularly hydrocarbons, garnered significant attention during the bioremediation phase of the study due to their critical relevance. Furthermore, to guarantee ecological value and minimize the impact of non-native species, only indigenous microbial populations from the wetlands were used. After being carefully examined, the samples verified their validity and applicability to real environmental pollution levels.
2.1.2. Exclusion Criteria
The study excluded sites devoid of verified anthropogenic pollution or those inaccessible for logistical or environmental considerations. None of the microbiological samples were contaminated with anything other than bacteria or had problems with the experiment, including malfunctioning equipment or low DNA quality, thus maintaining the accuracy and integrity of the microbiological and environmental studies.
2.2. Sample Collection
For this experimental study, water and sediment samples were gathered from five distinct sites within the Mesopotamian marshes during the dry season of 2024. Agricultural runoff, urban wastewater, and industrial discharge were among the known sources of contamination; therefore, these test locations were especially selected. This approach ensured that the gathered samples fairly reflected the degrees of contamination in the marsh.
2.2.1. Sampling Sites and Geographic Coordinates
The sampling sites and their geographic coordinates are listed as follows:
1. Central marshes(31.25°N, 47.17°E): located near Amarah and exposed to agricultural runoff and urban effluents.
2. Hammar marshes(30.89°N, 46.60°E): situated near Nasiriyah and influenced by the Euphrates River and local agricultural activities.
3. Hawizeh marshes(31.50°N, 47.75°E): receiving water from the Tigris distributaries and potentially impacted by cross-border pollutants.
4. Southern Hammar marshes(30.50°N, 47.10°E): located near Basra and exposed to urban and industrial pollution.
5. Northern Hawizeh marshes(31.65°N, 47.40°E): existing near Al-Musharrah and reflective of northern marsh conditions.
2.2.2. Sample Size
This study gathered five sediment samples (one from each of the five sites) and ten water samples overall (two from each of the five sites) to ensure representative geographical coverage over the Mesopotamian marshes. Double water samples were gathered from every site to reflect its geographic variety. This ensured the reproducibility and accuracy of the data. The microbial substrate of the bioreactor experiment was a composite sample created from homogenized and pooled sediment samples from all sites. The sample size and collection strategy were determined based on past bioremediation studies in similar aquatic environments, which have shown that sampling from three to five representative sites with replicates is sufficient for analyzing pollution distribution patterns and microbial community structures. This approach ensured a comprehensive spatial representation of pollution sources, facilitated laboratory processing and analysis, and preserved the study's applicability to the objectives of environmental restoration.
2.2.3. Sample Handling
To prevent the degradation of the samples by microorganisms or chemicals during their transportation to the laboratory, they were collected in sterile containers, immediately placed in the refrigerator, and subjected to rigorous environmental controls. Water samples were tested directly, while sediment samples were retained for reactor construction. The objectives of this study aligned with this type of controlled sampling, as they involved testing the effectiveness of hydrocarbon biodegradation utilizing natural microbial communities from various marsh environments.
2.3. Experimental Reactor Setup
A packed-bed reactor built with 100 grams of rich microbial marsh sediments as the substrate was used to assess hydrocarbon biodegradation. Designed for simple viewing, the see-through acrylic reactor housed 1 litre of water samples gathered from the sites indicated in the sampling portion. Keeping the C: N:P ratio of 100:10:1, the substrate was prepared with a nutrient solution of ammonium nitrate and potassium phosphate, therefore improving the native microbial population, before the experiment. To start the process, the silt was left to incubate in the nutrient solution at room temperature for 48 hours to promote microbial development. The packed bed reactor was then configured to operate in batch flow mode, allowing contaminated water to settle in the sediment layer. To guarantee enough oxygen for aerobic biodegradation and aerate the reactor, a fine bubble diffuser was positioned at its base. A temperature control system was used to keep the temperature between 25 and 30 degrees Celsius and the pH levels between 6.8 and 7.2, which is the best range for microbial activity. Samples from the reactor were obtained every 24 hours to examine hydrocarbons by gas chromatography-mass spectrometry. This design made it possible to carefully investigate hydrocarbon breakdown using the naturally occurring microbial population from marsh sediments. The reactor radius was 20 cm, and the height was 100 cm. The reactor was aerated continuously during the experiment using an air pump connected to a tube that fitted at the bottom of the reactor in a spiral shape, and that tube had a nozzle every 2 metres to distribute the air effectively. The marsh sediment was distributed above the air spiral tube. Degradation was confirmed to be microbially mediated based on the results obtained from GC-MS analysis under abiotic control (e.g., sterilized sediment) in the bioreactor experiments.
2.4. Analytical Techniques
The presence of organic pollutants was analyzed using GC-MS. Samples were prepared by extracting pollutants using solid-phase microextraction techniques. The GC-MS analysis allowed for the precise identification and quantification of various organic compounds in the water samples. The minimum detectable limit of the GC-MS is likely between 0.001 and 0.008 ppm.
2.5. Molecular Analysis
Genomic DNA was extracted from both pretreatment (before bioremediation) and posttreatment (after bioremediation) sediment samples using a commercial DNA extraction kit, following the manufacturer’s instructions to ensure high-quality DNA suitable for downstream applications. The 16S rRNA gene was amplified using universal primers targeting the V3-V4 region (341F: 5-CCTACGGGNGGCWGCAG-3 and 805R: 5-GACTACHVGGGTATCTAATCC-3) in a polymerase chain reaction (PCR) setup.
Recent studies have highlighted the importance of precise primer selection in identifying hydrocarbon-degrading bacteria. For instance, primers, such as those targeting regions similar to our setup, are commonly used in environmental microbiology studies. The PCR conditions included initial denaturation at 95°C for 3 minutes, followed by 25 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 1 minute, concluding with a final extension at 72°C for 10 minutes. The amplified products were verified by gel electrophoresis. Subsequently, amplicons were subjected to sequencing. The DNA sequence was aligned with closely related bacterial species obtained from the NCBI database. Finally, a phylogenetic tree was constructed to illustrate the relationship among related species [16].
3. RESULTS AND DISCUSSION
The Mesopotamian marshes, recognized as a UNESCO world heritage site, hold significant ecological and cultural importance, but they currently face severe pollution pressures from agricultural runoff, oil extraction, and urban discharge. Their ecological restoration is, therefore, regarded as both a national priority and a global environmental concern. To strengthen their importance, the unique microbial diversity of the marshes, hypothesized to play a vital role in bioremediation, has been highlighted. In addition, detailed water properties and pollutant concentration data have been provided in this work (Table 1), along with site-specific descriptions. For example, the Central marshes have been shown to contain the highest levels of contamination, including aliphatic hydrocarbons (100 µg/L), phenols (40 µg/L), aromatic hydrocarbons (5000 µg/L), and pesticides (120 µg/L). These additions provide a clear rationale for selecting Mesopotamian marsh water and emphasize the urgency of developing effective bioremediation strategies for this ecosystem.
The analysis of the water samples shown in Table 1 reveals varying levels of pollutants across different marshes in Iraq. The concentrations of aliphatic hydrocarbons, phenols, aromatic hydrocarbons, and pesticides have been measured in several marshes, including the Central marshes, Hammar marshes, Hawizeh marshes, and Northern Hawizeh marshes.
The Hammar marshes provided two datasets. The first set consisted of aliphatic hydrocarbon concentrations of 60.0 µg/L, phenol concentrations of 25.0 µg/L, aromatic hydrocarbon values of 2500 µg/L, and pesticide concentrations of 75.0 µg/L. Though another set of findings revealed aliphatic hydrocarbons at 70.0 µg/L, aromatic hydrocarbons at 3500 µg/L, and pesticides at 90.0 µg/L, the amount of phenols was not stated. These variations suggested that pollution levels can vary significantly even within a single marsh, maybe in response to regional factors as agricultural runoff or industrial activities [17]. The Southern Hawizeh marshes had lower pollution concentrations: 20.0 µg/L for aliphatic hydrocarbons, 10.0 µg/L for phenols, 1200 µg/L for aromatic hydrocarbons, and 30.0 µg/L for pesticides. The Northern Hawizeh marshes showed rather lower concentrations of aliphatic hydrocarbons (40.0 µg/L), aromatic hydrocarbons (2000 µg/L), and pesticides (50.0 µg/L), even though the phenol levels were not reported.
The levels of organochlorine pesticides, such as DDT and endrin, varied significantly between sites depending on prior use and agricultural runoff. Most aliphatic hydrocarbons result from both natural and synthetic sources. Either pollution or naturally occurring mechanisms might lead to the presence of phenolic compounds in ecosystems and water sources.
| Site | Aliphatic Hydrocarbons (µg/L) | Phenols (µg/L) | Aromatic Hydrocarbons (µg/L) | Pesticides (µg/L) |
|---|---|---|---|---|
| 1 | 100.0 | 40.0 | 5000 | 120.0 |
| 2 | 60.0 | 25.0 | 2500 | 75.0 |
| 3 | 20.0 | 10.0 | 1200 | 30.0 |
| 4 | 70.0 | Not specified | 3500 | 90.0 |
| 5 | 40.0 | Not specified | 2000 | 50.0 |
Typically associated with petroleum products and industrial activities, areas closest to urban or industrial sources exhibit higher concentrations of aromatic hydrocarbons [18]. Since pollution concentrations varied throughout different marshes, concentrated management solutions are needed to solve issues in these habitats. Polluted sites, like the Central marshes [19], threaten both human health and wildlife. Contaminants [5] can affect the ecological purposes of these wetlands, including preserving biodiversity and pollution filtration. The measured pollution levels have been found to emerge from several different sources. Among the anthropogenic activities projected to be somewhat important are the production and refining of oil, agricultural runoff, and the lack of wastewater treatment [20]. The deliberate drying up of marshes in the past has aggravated present pollution issues and also reduced their natural capacity to filter pollutants [21].
Figure 1 presents the changes in concentrations of the organic contaminants found in the treated water from the Central marshes (site 1). Figure 1A demonstrates that aromatic hydrocarbon concentrations decreased from 5000 to 800 µg/L, indicating an 84% reduction over seven days. This significant decrease suggests that these compounds are also highly susceptible to degradation or removal. Figure 1B demonstrates the changes in concentrations of aliphatic hydrocarbons, phenols, and pesticides. Figure 1B reveals a consistent decrease in the concentrations of aliphatic hydrocarbons, phenols, and pesticides over seven days. The concentration of aliphatic hydrocarbons decreased significantly from 100 µg/L on day 1 to 14.3 µg/L on day 7, indicating an 85.7% reduction. This rapid decrease indicated these compounds as highly susceptible to degradation or removal processes. Phenol concentrations decreased by 60% (from 40 to 16 µg/L) over the study period. Although this reduction was notable, it was less pronounced compared to that of aliphatic hydrocarbons and aromatic hydrocarbons, suggesting that phenols may be more resistant to degradation. Pesticide concentrations decreased by 50% (from 120 to 60 µg/L) over the study period. This reduction has been found to be consistent but less pronounced compared to that of aromatic and aliphatic hydrocarbons, indicating that pesticides may be more persistent in the environment. This finding has also been confirmed by O’Donnell and his research group [16].
Figure 2 presents the changes in concentrations of organic contaminants in treated water from the Hammar marshes (site 2), showing a rapid decrease in pollution levels. Figure 2A demonstrates that aromatic hydrocarbon concentrations decreased from 2500 to 200 µg/L, indicating a 92% reduction. Figure 2B demonstrates the changes in concentrations of aliphatic hydrocarbons, phenols, and pesticides. The concentration of aliphatic hydrocarbons decreased significantly from 60 µg/L on day 1 to 0.1 µg/L on day 7, indicating a nearly complete elimination of these compounds. This rapid decrease suggested aliphatic hydrocarbons to be highly susceptible to degradation or removal processes. Phenol concentrations decreased from 25 to 12.5 µg/L over the study period, indicating a 50% reduction. However, a slight increase was observed on day 7, suggesting a variability in degradation rates or environmental conditions. Pesticide concentrations decreased from 5 to 2 µg/L over the study period, indicating a 60% reduction. This consistent decrease indicated that pesticides can be effectively removed or degraded.
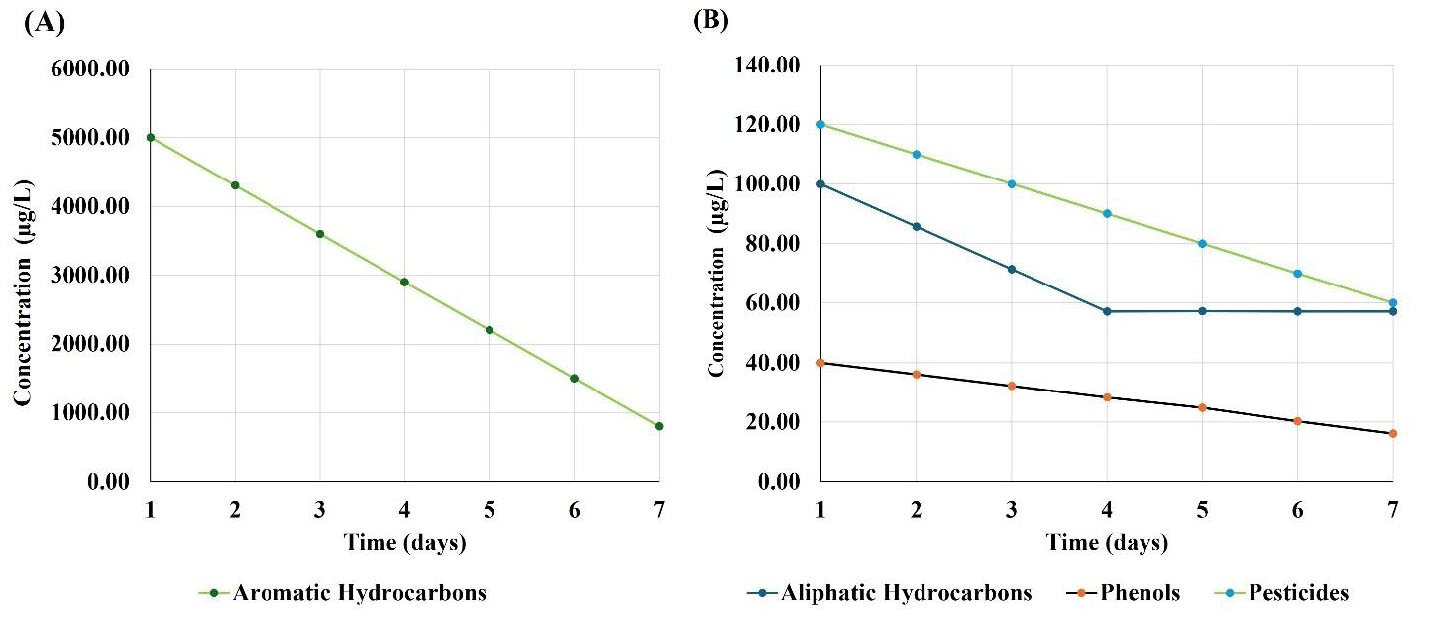
Changes in concentrations of the organic contaminants found in the treated water from the Central marshes (site 1). The x-axis represents time (days), and the y-axis represents contaminant concentration (µg/L). (A) Aromatic hydrocarbons; (B) aliphatic hydrocarbons, phenols, and pesticides.
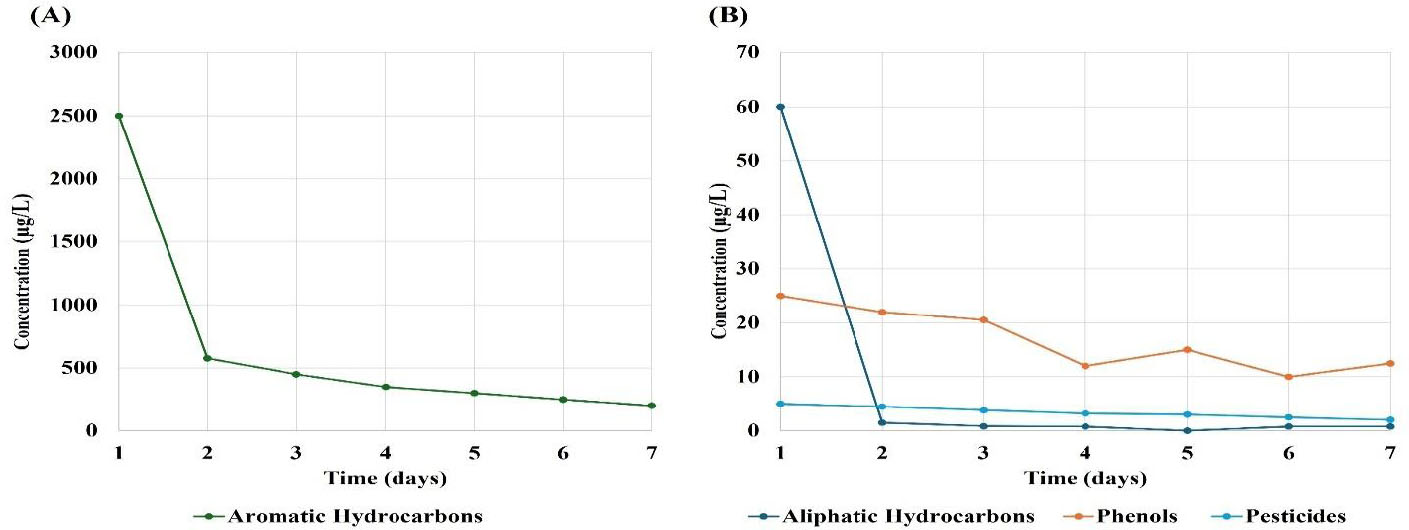
Changes in concentrations of the organic contaminants found in the treated water from the Hammar marshes (site 2). The x-axis represents time (days), and the y-axis represents contaminant concentration (µg/L). (A) Aromatic hydrocarbons; (B) aliphatic hydrocarbons, phenols, and pesticides.
Figure 3 presents the changes in concentrations of organic contaminants in treated water from the Hawizeh marshes (site 3). Figure 3A demonstrates that aromatic hydrocarbon concentrations dropped significantly from 1200 to 250 µg/L, indicating a 79% decrease. This substantial reduction indicated these compounds to also be highly susceptible to degradation or removal processes. Figure 3B demonstrates the changes in concentrations of aliphatic hydrocarbons, phenols, and pesticides. The concentration of aliphatic hydrocarbons plummeted from 20 µg/L on day 1 to less than 0.1 µg/L on day 7. This near-total elimination indicated aliphatic hydrocarbons to be highly susceptible to degradation or removal. Phenol levels decreased from 10 to 3.5 µg/L over the study period, indicating a 65% reduction. This consistent decrease suggested that phenols can be effectively degraded or removed, although at a slower rate compared to that of aliphatic hydrocarbons. Pesticide concentrations decreased from 30 to 15 µg/L, indicating a 50% reduction. This consistent decrease suggested that pesticides can be effectively managed, although they remain present in the environment [16].
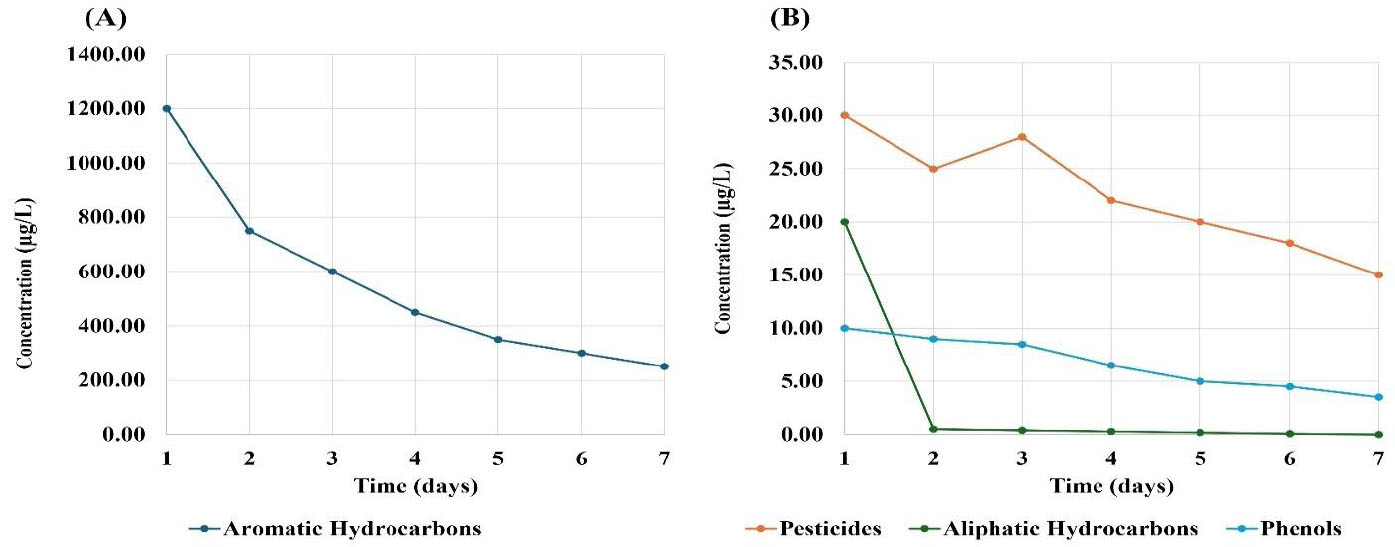
Changes in concentrations of the organic contaminants found in the treated water from the Hawizeh marshes (site 3). The x-axis represents time (days), and the y-axis represents contaminant concentration (µg/L). (A) Aromatic hydrocarbons; (B) aliphatic hydrocarbons, phenols, and pesticides.

Changes in concentrations of the organic contaminants found in the treated water from the Southern Hammar marshes (site 4). The x-axis represents time (days), and the y-axis represents contaminant concentration (µg/L). (A) Aromatic hydrocarbons, aliphatic hydrocarbons, and pesticides (from a concentration scale of 0 to 4000 µg/L); (B) aliphatic hydrocarbons and pesticides (from a concentration scale of 0 to 100 µg/L).
Figure 4 presents the changes in concentrations of organic contaminants in treated water from the Southern Hammar marshes (site 4). Figure 4A demonstrates that the concentrations of aliphatic hydrocarbons, aromatic hydrocarbons, and pesticides notably decreased over the study period from a concentration scale of 0 to 4000 µg/L. Aliphatic hydrocarbons underwent a precipitous decrease, plummeting from an initial 70 µg/L on day 1 to below the detection threshold of 0.1 µg/L on day 5, indicating high susceptibility to degradation or removal mechanisms. Aromatic hydrocarbon concentrations decreased from 3500 to 400 µg/L, corresponding to an 88.6% reduction, suggesting effective degradation or removal processes. Meanwhile, pesticide concentrations decreased consistently from 90 to 55 µg/L, indicating a 38.9% reduction, suggesting effective management, although they persisted in the environment at reduced levels. Figure 4B demonstrates the concentrations of aliphatic hydrocarbons and pesticides from a scale of 0 to 100 µg/L.
Figure 5 presents the changes in concentrations of organic contaminants in treated water from the Northern Hawizeh marshes (site 5). Figure 5A illustrates the concentrations of aromatic hydrocarbons, which significantly decreased over the study period. Aromatic hydrocarbons also showed a substantial decrease, dropping from 2000 to 400 µg/L, indicating an 80% reduction, thereby suggesting effective degradation or removal processes. Figure 5B illustrates that the concentrations of aliphatic hydrocarbons and pesticides significantly decreased over the study period. Aliphatic hydrocarbon concentrations demonstrated a rapid reduction, decreasing from 40 µg/L on day 1 to below the detection threshold of 0.1 µg/L on day 5, indicating high susceptibility to degradation or removal processes. Meanwhile, pesticide concentrations decreased consistently from 50 to 35 µg/L, indicating a 30% reduction, suggesting effective management, although they persisted in the environment at reduced levels. These trends collectively revealed that degradation effectiveness is associated with the types and complexity of the organic compounds involved [22].
The phylogenetic analysis of the bacteria isolated from the reactor substrate after treatment revealed unidentified bacterial species labeled as FF-A5 and FF-A6, as shown in Figure 6. These bacterial species have been found to be involved in marsh pollutant biodegradation. As shown in Figure 6, FF-A5 and FF-A6 were in a distinct clade from all other species in the phylogenetic tree, suggesting that these bacterial species probably have an essential role in organic compound biodegradation. Furthermore, these species are considered indigenous genera in the Mesopotamian marsh sediments. The phylogenetic analysis in Figure 6 shows that the isolated species were located between two subclades of Pseudomonas and Clostridium. This analysis compared several key bacterial genera with effective organic bioremediation potential. These undefined bacterial species (FF-A5 and FF-A6) may possess an effective degradation pathway that mineralizes the targeted organic compounds, as indicated by the results of the batch reactor experiment. The expected degradation pathway may be closely correlated with the nearest clusters of Pseudomonas and Clostridium (Fig. 7) [22].
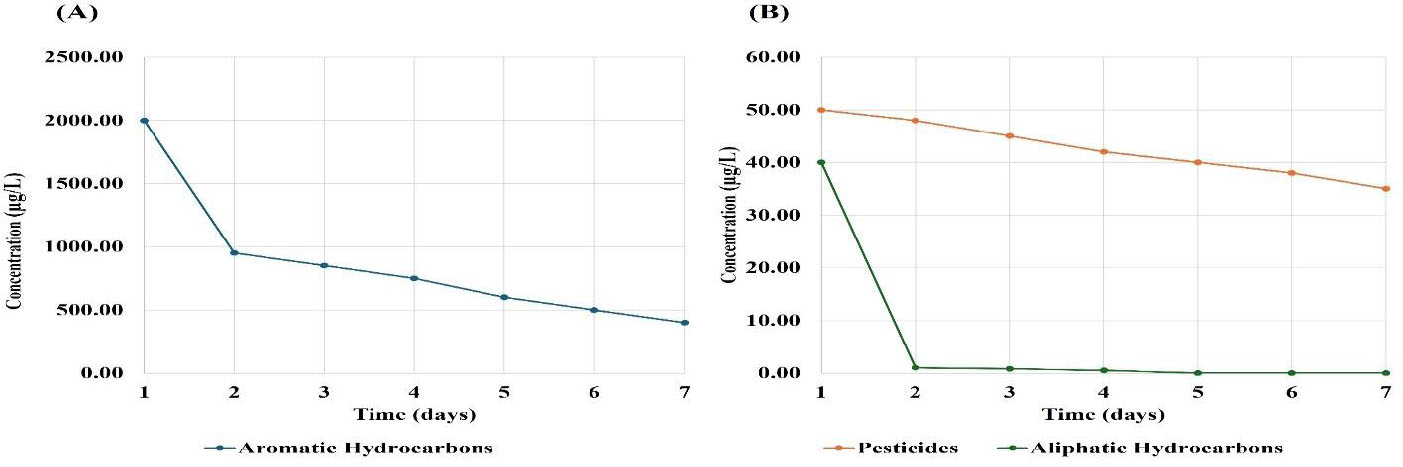
Changes in concentrations of the organic contaminants found in the treated water from the Northern Hawizeh marshes (site 5). The x-axis represents time (days), and the y-axis represents contaminant concentration (µg/L). (A) Aromatic hydrocarbons; (B) aliphatic hydrocarbons and pesticides.
Table 2 shows the genes involved in hydrocarbon compound degradation. In Pseudomonas spp., several operons, such as xyl, tod, cat, nah, and ben, play a major role in breaking down hydrocarbons, aromatics, and other organic compounds in soil, water, and industrial waste [23]. A previous study highlighted that Clostridium species significantly contribute to recalcitrant pollutant degradation [24]. Furthermore, recent metagenomic studies [25] have demonstrated that Pseudomonas species often dominate bioremediation systems used for treating complex pollutant mixtures because of their extensive degradative enzyme systems and metabolic versatility. Particularly notable is the presence of P. nitroreducens, which has been specifically linked to the degradation of aromatic compounds and nitroaromatic pollutants in a recent work [26]. Another study reported a comparable assemblage of Bacillus and Pseudomonas species used in the treatment of petroleum hydrocarbons in constructed wetlands. However, in this study, the labeled bacterial species were closely related to Pseudomonas. The role of Pseudomonas species in the bioremediation of organic pollutants was demonstrated by Kashani and his team [27]. In addition, Ivanova reported that Pseudomonas-dominated consortia achieved higher mineralization rates for recalcitrant aromatic compounds compared to single-strain systems. It was also observed that Pseudomonas genomic analysis revealed that cross-feeding interactions among different Pseudomonas species enhanced the overall degradation efficiency, which may explain the diverse Pseudomonas population in our reactor substrate [23]. The previously reported finding confirmed that the use of synergism among multiple bacterial species can accelerate the bioremediation process, as effectively used in this study.
A recent work by Sarsan et al. on batch bioreactors used for treating industrial effluents found that microbial synergism among various bacterial species, such as Bacillus and Pseudomonas species, could enhance the bioremediation process [28]. In addition, the use of ingenious microbial genera improved biodegradation rates in batch systems [29].
| Gene Family | Function | Key Genes |
|---|---|---|
| xyl operon | Degradation of toluene and xylene |
xylA – toluene monooxygenase xylB – benzyl alcohol dehydrogenase xylC, xylD, etc. – enzymes for further degradation steps |
| cat operon | Breakdown of catechol, a key intermediate in aromatic compound metabolism |
catA – catechol 1,2-dioxygenase (cleaves catechol) catB, catC – downstream metabolic enzymes |
| tod operon | Breakdown of catechol, a key intermediate in aromatic compound metabolism |
catA – catechol 1,2-dioxygenase (cleaves catechol) catB, catC – downstream metabolic enzymes |
| nah operon | Naphthalene degradation |
nahA to nahF (upper pathway) nahG to nahL (lower pathway) |
| ben operon | Degradation of benzoate | benA, benB, benC, etc. |
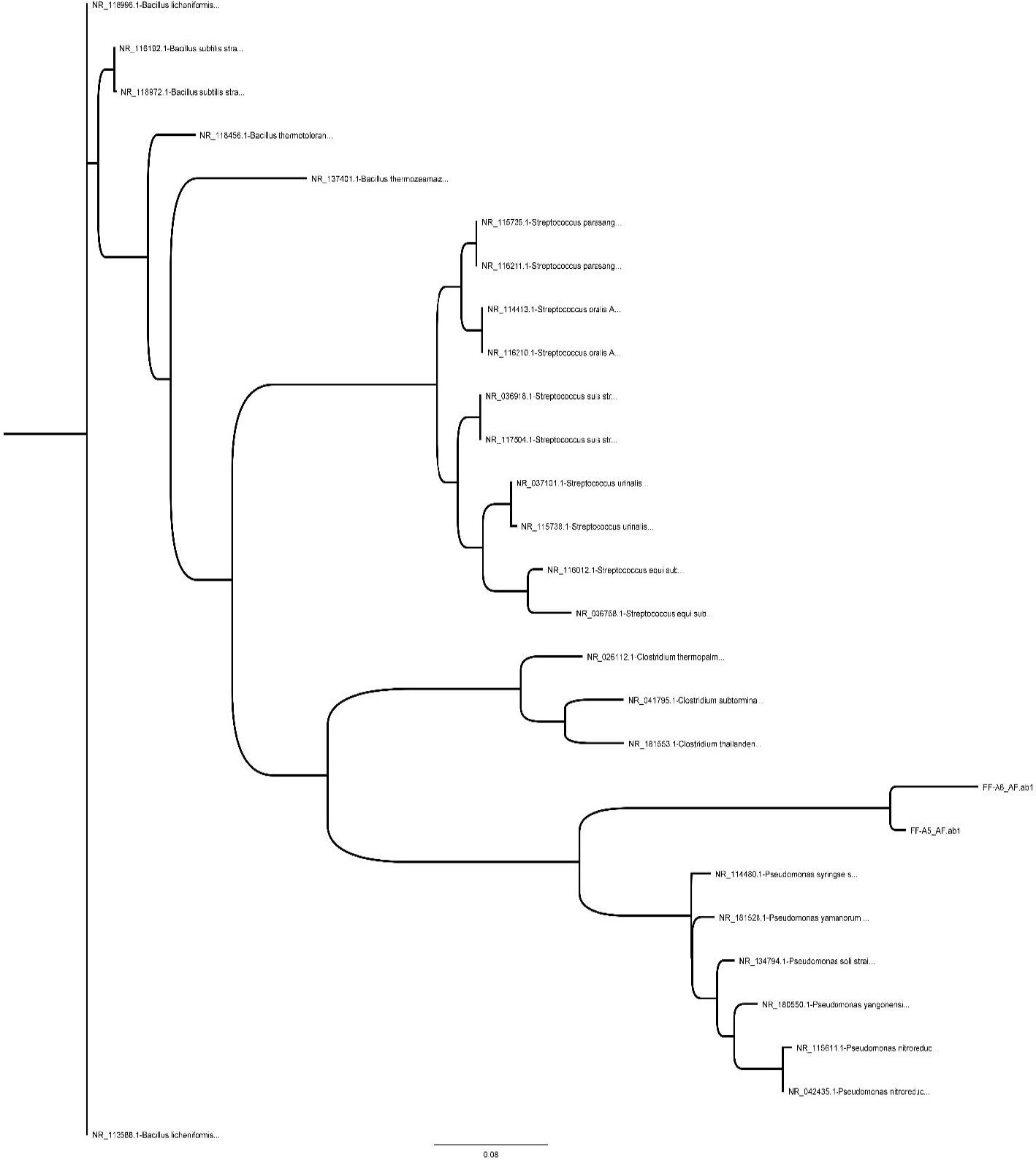
Phylogenetic tree for the reactor substrate.
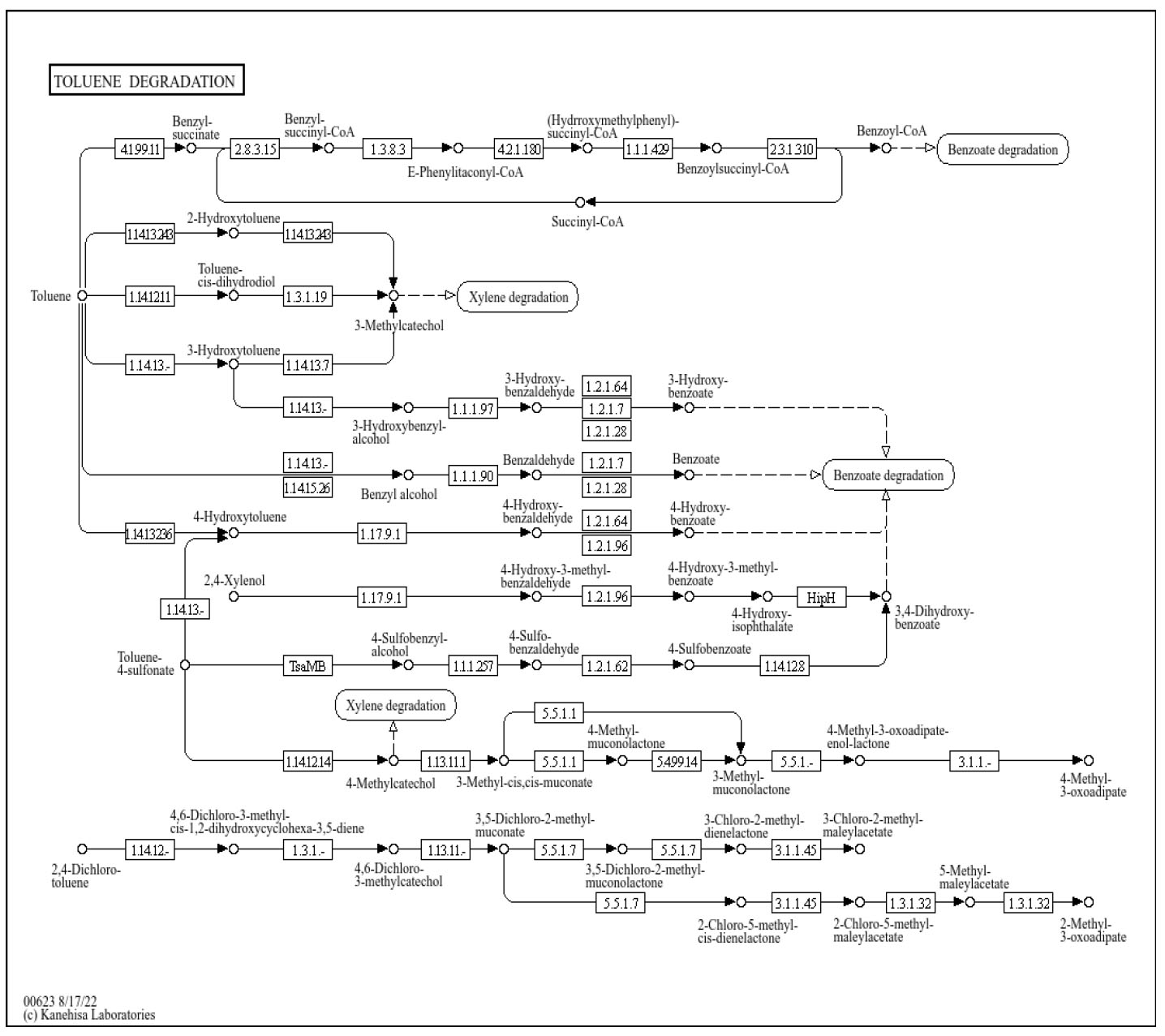
Degradation pathways of organic compounds in Pseudomonas with key genes.
Our results were compared with the existing literature and are presented in the supplementary materials. The novelty of our study lies in several key contributions that distinguish it from previous bioremediation research. In the discipline of environmental microbiology, the Mesopotamian marshes of Iraq have not been researched extensively. This work has been the first to use natural microbial communities from that area to break down several types of organic contaminants, like pesticides, aliphatic and aromatic hydrocarbons, and phenols. Second, two bacterial strains that had not been detected before, FF-A5 and FF-A6, have been found to be quite common in the microbial community after treatment. These strains are likely to play an important part in breaking down contaminants. Since these strains have never been associated with breaking down hydrocarbons before, they may have new catabolic capacities. Third, we were able to fully assess the effectiveness of bioremediation and keep track of changes in microbes in response to contamination using a controlled batch bioreactor and molecular approaches (16S rRNA gene profiling). All of these parts together make it possible for possible field-scale applications and can help us learn more about the native biodegradative ability in a wetland ecology that is vital to the environment, but has not been researched much.
Our phylogenetic investigation discovered two new bacterial strains, FF-A5 and FF-A6, which were very important to the biodegradation process in the batch bioreactor. It was observed that strain FF-A6 was more metabolically active at breaking down aromatic and aliphatic hydrocarbons over time, and they were extremely effective at breaking down pollutants. A “best” strain cannot be identified until further genomic, enzymatic, or functional tests are performed on the two strains, as they have not been fully described yet. Studying these strains in pure cultures can assist future studies in figuring out how well they break down pollutants and if they have a synergistic effect.
The findings of this study further demonstrated that the field of bioremediation can be advanced through the use of indigenous microbial communities from the Mesopotamian marshes, an ecosystem that has been largely overlooked in previous research. Rapid and substantial mineralization of organic pollutants was achieved, with reductions ranging from 79% to 99% within seven days. In addition, two novel bacterial strains, FF-A5 and FF-A6, were identified and shown to possess significant degradation capabilities. The merit of this method lies in its sustainability and ecological relevance. Because native microbial consortia were utilized, ecological risks associated with the introduction of non-native species were minimized, while the potential for successful large-scale application was enhanced. By demonstrating both efficiency and ecological compatibility, this approach may provide valuable insights into how native microbial systems can be integrated into long-term strategies for environmental restoration.
In this study, the focus was placed on organic pollutants, as they represent the most immediate threats to the Mesopotamian marshes and are closely associated with oil extraction, agricultural runoff, and industrial discharge. By concentrating on hydrocarbons, phenols, and pesticides, a foundation was established for understanding how native microbial communities contribute to bioremediation in this ecosystem. Through the use of chemical analysis (GC-MS) and molecular tools (16S rRNA sequencing), reductions in these contaminants were documented, and insights into the microbial dynamics driving the process were obtained, including the identification of novel strains with strong degradation potential. While this targeted approach generated valuable findings, it also meant that other pollutants, such as heavy metals and nutrients, have not been addressed. This limitation has been acknowledged, and it is suggested that future investigations expand to include these additional contaminants to provide a more comprehensive picture of the marshes’ bioremediation potential.
Finally, the study discovered that mineralization occurred when organic pollutants, such as aliphatic hydrocarbons, phenols, aromatic hydrocarbons, and pesticides, decreased significantly. It is believed that the microbes in the marsh sediments, especially the two most abundant types of bacteria, FF-A5 and FF-A6, assisted in breaking down these contaminants. Like other mineralization processes, this one probably broke down the organic pollutants into inorganic compounds, like water and carbon dioxide [30]. The statistics, which indicated an 85% to 99% drop in aliphatic hydrocarbons and a 79% to 92% drop in aromatic hydrocarbons, provided more proof that mineralization transpired following treatment. According to a phylogenetic examination of the microbial species found in the batch reactor, these bacteria, which are probably native to marshy areas, have an amazing ability to mineralize organic pollutants. The fact that pollution levels declined downward indicated that these chemicals have been turned into minerals, meaning that the marsh ecosystem has been restored and broken down.
CONCLUSION
This research work has demonstrated that indigenous microbial communities in Mesopotamian wetlands may digest organic pollutants in a batch bioreactor. Within just seven days, substantial reductions were accomplished in aliphatic hydrocarbons (reduced to 99%), aromatic hydrocarbons (reduced to 92%), phenols (reduced to moderate levels), and pesticides (reduced to moderate levels). Initially, the Central marshes exhibited the highest levels of contamination. The exceptional breakdown efficiency was likely attributable to two newly identified bacterial strains, FF-A5 and FF-A6, discovered by phylogenetic research. These strains have been found to be closely associated with Pseudomonas and Clostridium. The findings have indicated that microbial synergy and indigenous microbial consortia can serve as effective mechanisms for the enduring regeneration of damaged wetland ecosystems.
This paper has elucidated the bioremediation alternatives for marsh sediments in the treatment of organic contamination, despite specific limitations. It is important to note that the experiment was performed in a controlled laboratory setting, which may not accurately represent the complexity and unpredictability of real marsh ecosystems. Even if this sample was representative, site-specific microbial diversity and localized degradation mechanisms were probably neglected due to the singular composite sediment sample. Furthermore, limiting the sampling to the dry season of 2024 may have excluded the assessment of the effect of seasonal variations on microbial activity and pollution levels. Additionally, the study utilized GC-MS and 16S rRNA analyses, which, while beneficial, may not have detected low-abundance chemicals or microbial taxa that are essential for the environment. The lack of long-term monitoring may have complicated the evaluation of the enduring efficacy and ecological impacts of the bioremediation technique. Future research must incorporate in situ studies, seasonal sampling, and comprehensive molecular analysis to validate scalability and substantiate the conclusions.
The results of this study should be regarded as preliminary. The lab-scale results, however, have been found to be promising for using native microbial communities to break down organic pollutants. When tested in real life, results can be very different due to various factors, like site-specific conditions, competition amongst microbes, and changes in water flow. Therefore, future pilot-scale and field-based validation trials must be conducted before recommending large-scale implementation of this bioremediation approach. Researchers will continue to study the bacterial genera in more depth, looking for the genes involved and providing insights into the enzymes and breakdown processes associated with them.
AUTHORS’ CONTRIBUTIONS
S.A.A.: Contributed to the study plan and experiment design; G.M.A.: Experiments were carried out; A.M.M.: Data analyses were carried out; S.A.A. and G.M.A.: Manuscript was first drafted; A.M.M.: Edited and critically revised. All authors read and approved the final version of the manuscript.
AVAILABILITY OF DATA AND MATERIALS
All data generated or analyzed during this study are included in this published article. Also, the materials and data on any of the plants used in the current study will be made available by the corresponding author upon reasonable request.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Institute of Genetic Engineering and Biotechnology for Postgraduate Studies, University of Baghdad, Baghdad, Iraq.


