All published articles of this journal are available on ScienceDirect.
Cam β-NGF Supplementation: A Promising Approach to Preserve Dromedary Camel Sperm Quality During Short-term Storage
Abstract
Introduction
The role of camel βeta-Nerve Growth Factor protein (Cam β-NGF), isolated from camel seminal plasma, in preserving sperm quality remains poorly defined. This study aimed to evaluate the impact of Cam β-NGF on mass motility, viability, and membrane integrity in dromedary (Camelus dromedarius) semen stored for 24 hours at 4 °C.
Methods
The semen samples were treated with varying concentrations of Cam β-NGF (10, 100, 500, and 1000 ng/mL) after preservation. Ten semen samples with mass motility ≥ 3 and viability ≥ 60% were selected, washed, suspended in HBSS, and treated with TCF-EY. After 24 hours of storage at 4 °C, samples were maintained at 36 °C with Cam β-NGF supplementation for 60 minutes. Mass motility, viability, and membrane integrity were assessed at T0, T30, and T60 minutes.
Results
The results revealed a significant decline in sperm quality traits after 24 hours of storage at 4 °C. However, mass motility was conserved after 30 minutes of incubation with 1000 ng/mL and 500 ng/mL of Cam β-NGF (p < 0.05). On the other hand, viability was significantly higher after 60 minutes of incubation with 1000, 500, and 100 ng/mL of Cam β-NGF compared to the control extender and 10 ng/mL supplementation (p < 0.05). Additionally, membrane integrity was significantly maintained at T30 and T60 minutes with 1000, 500, and 100 ng/mL of Cam β-NGF (p < 0.05).
Discussion
Short‐term storage at 4 °C markedly reduced dromedary camel sperm motility, viability, and membrane integrity, consistent with cold‐induced oxidative stress. Adding purified Cam β-NGF dose-dependently preserved these parameters over 1 h of incubation, indicating its value as a protective supplement for semen storage.
Conclusion
Cam β-NGF helps protect and maintain sperm traits during short-term storage; it seems to reduce alterations that might lower sperm quality. Thus, supplementing semen with Cam β-NGF may help maintain sperm quality, offering potential benefits for artificial insemination programs in dromedaries.
1. INTRODUCTION
Cryopreservation of sperm and the development of assisted reproductive technologies are widely used techniques for preserving and supplying sperm, supporting breeding programs, and maintaining genetic diversity in livestock and wildlife. However, dromedary camel sperm is typically regarded as low quality, exhibits high viscosity that complicates handling, dilution, and evaluation, and is not tolerant to freezing and thawing procedures [1, 2]. Besides, attempts to cryopreserve dromedary camel spermatozoa have been met with reduced success. The cryopreservation process begins with dilution with an extender (36°C), followed by a cooling step (4–5°C), the addition of the cryoprotectant, equilibration, and then freezing in liquid nitrogen at −196°C. Each step of this process, along with thermal changes, can cause gamete damage, the extent of which remains unknown. However, it has been reported that using some extenders in the process of artificial insemination affects sperm motility within 12–24 hours of cold storage [3]. The composition of camel seminal plasma appears to be particular. Camelids have higher levels of βeta-Nerve Growth Factor (β-NGF) than other mammals [4]. This protein, a member of the neurotrophin family, plays a crucial role not only in the nervous system but also in other non-nervous systems, such as the reproductive system [5]. In dromedary seminal plasma, Fatnassi et al. [6] detected the presence of the β-NGF named “Cam-β-NGF” with a molecular weight of approximately 14 kDa. This protein induced ovulation at rates similar to GnRH. Nerve growth factor is likely one of the most widely researched neurotrophins [7]. Numerous studies have demonstrated its presence in germinal cells at every developmental phase.
Furthermore, the effects of NGF on reproductive systems and sperm function have recently been assessed in animal models [8-10]. These studies highlight its role in supporting testis formation and development, as well as influencing sperm differentiation, maturation [11, 12], viability [9], motility [13], and DNA integrity in humans [14]. This study aimed to investigate the effect of Cam β-NGF addition on the motility, viability, and membrane integrity of dromedary (Camelus dromedarius) semen during short-term cold storage (24 h at 4 °C).
2. MATERIALS AND METHODS
2.1. Animal Location
Six clinically healthy male dromedary camels (Camelus dromedarius), aged between 8 and 15 years and with an average body weight of 575.3 ± 32.2 kg, were used in this study. These camels were in good condition, with a body condition score of 4.0 ± 0.4 (on a scale from 0 to 5 [15]). They were raised at the Arid Lands Institute’s artificial insemination center in Medenine, Tunisia (33° 30′ N, 10° 40′ E), under intensive management conditions. Each male was kept individually in a box measuring 3 m in height, 5 m in length, and 3m in width with a sand floor. In front of each box, the animals had free access to an open area throughout the day to minimize stress caused by confinement. The camels' diet included 4.5 kg of oat hay distributed at 9:00 a.m., along with a 4 kg concentrate supplement composed of barley, wheat bran, olive cake, minerals, and vitamins, provided at 3:00 p.m. Water was made available every 48 hours. A 10-year-old female was present as a behavioral stimulus during semen collection with an artificial vagina; no mating occurred, no procedures were performed on her, and she was not a research subject in this study (as shown in Section 2.2).
2.2. Semen Collection and Ejaculate Evaluation
Semen was collected twice a week with a bovine artificial vagina (IMV, France) in the presence of a female camel restrained in sternal recumbency on padded flooring (cushioned position) to prevent injury [16]. The female was used solely to stimulate mounting; no mating or invasive procedures were performed, and all procedures were supervised by a veterinarian. All animal procedures were approved by the Animal Ethics Committee of the National School of Veterinary Medicine (AEC/IACUC, ENMV–Sidi Thabet, Tunisia; approval code CEEA-ENMV 64/22, approved on 26 December 2022). Semen collection was programmed with a 30 min maximal mounting time. After collection, semen volume was measured using a graduated collection tube. Then, 30 min after warming in a water bath at 36°C, mass motility, viability, membrane integrity, and concentration were determined. Mass motility (0–5) was evaluated by observing 50 μL of semen on a pre-warmed slide under a phase-contrast microscope (Nikon E200; Tokyo, Japan) at 200× magnification [17]. The proportion of viable spermatozoa was assessed using eosin-nigrosin staining [18] after counting 200 spermatozoa. Sperm concentration was measured after 90 min of incubation using a Thoma hemocytometer. The total number of spermatozoa was calculated by multiplying the sperm concentration by the volume of the ejaculate (Table 1).
Table 1.
| Parameters | Values (Mean ± SD) |
|---|---|
| Mass Motility | 5 ± 0 |
| Viability (%) | 77.25 ± 3.91 |
| HOST (%) | 74.90 ± 8.73 |
| Concentration (x108) | 7.35 ± 4.79 |
| Total number of SPZ (x108) | 32.68 ± 21.82 |
Plasma membrane integrity was tested using a hypo-osmotic swelling test, as described by Ramu and Jeyendran [19]. Briefly, 10 µL of semen was mixed with 100 µL of hypo-osmotic solution (HOS: 1.5 mM trisodium citrate and 1.5 mM fructose) and incubated in the semen/HOS mixture for at least 30 min at 36°C. Subsequently, 10 µL of the solution was deposited onto a pre-warmed slide covered with a coverslip, and sperm were examined under a phase-contrast microscope at 400× magnification. Two hundred spermatozoa per slide were counted from four microscopic fields and expressed as percentages. The swollen spermatozoa were considered normal sperm cells (Fig. 1). Immediately following collection, semen samples were diluted with HBSS (25 mM HEPES, 130 mM NaCl, 5 mM KCl, 0.36 mM NaH2PO4, 0.49 mM MgCl2, and 2.4 mM CaCl2; pH 7.4, 290 mOsm/kg) at 37°C. Samples were then centrifuged at 800 × g for 10 min at room temperature to discard seminal plasma.
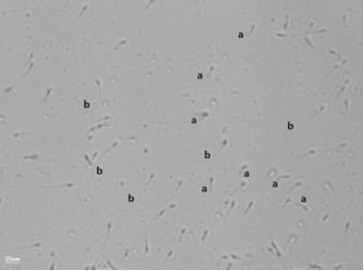
Hypo-osmotic swelling (HOS) test. (a) Spermatozoon with an intact plasma membrane, showing a swollen, coiled tail (normal). (b) Spermatozoon with a straight, unswollen tail (abnormal). Slides were prepared and examined under phase-contrast microscopy; images were captured with a Nikon E200 microscope fitted with a digital camera (400 × magnification).
2.3. Sperm Cooling and Conservation
Ten semen fractions were selected for short-term preservation with a mass motility score ≥ 3 and viability ≥ 60%. Sperm were kept at 36°C, and the results for concentration, sperm viability, mass motility, and swelling tests were evaluated as described above. The tris-citric acid-fructose-egg yolk extender (TCF-EY: 3.03% Tris, 1.2% citric acid, 1.7% fructose, 20% egg yolk, 1000 IU penicillin G sodium, and 1000 µg/mL gentamicin) was incorporated to reach a final concentration of 2 × 108 spermatozoa/mL. The samples were then transferred to a water bath at 36 °C. Samples were cooled (cooling rate 0.2 °C/min), then placed in a refrigerator (4–5 °C), and maintained for 24 h. Thereafter, preserved sperm cells were brought to 36°C in a water bath. Mass motility, viability, and membrane integrity were assessed after 15 min of warming.
2.4. Seminal Plasma Purification and Isolation of Cam-β-NG
Protein purification was conducted according to the method outlined by Fatnassi et al. [6]. Briefly, at room temperature and with a flow rate of 0.5 mL/min, 2 mL of camel seminal plasma (approximately 40 mg) was separated using fast protein liquid chromatography (FPLC; GE Healthcare Life Sciences, Bjorkgatan, Sweden) with a gel filtration column (SEC, Hi PrepTM 26/60 SephacrylTM S-100, GE Healthcare Life Sciences, Bjorkgatan, Sweden) (Fig. 2). The mobile phase used for elution consisted of phosphate-buffered saline with pH 7.4. This procedure resulted in the separation of two distinct protein peaks, which were subsequently pooled individually and stored at −20 °C until needed. The pool of the major peak (peak 2) was identified [6] as Cam-β-NG for the remainder of the experiment; it was desalted, lyophilized, and preserved at −20 °C until use.
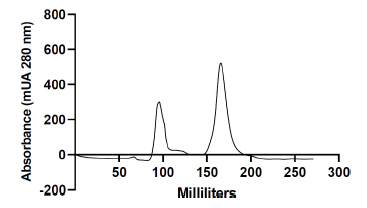
Protein chromatography profile of dromedary camel seminal plasma obtained after purification by fast protein size-exclusion liquid chromatography (FPLC).
2.5. Addition of Cam-β-NGF in Semen after 24 h of Conservation at 4°C
A minimum of 48–50% viable sperm and the absence of sperm agglutination were confirmed before starting the experiment. After warming, samples were fractionated into 5 aliquots and supplemented with different doses (10 ng/mL, 100 ng/mL, 500 ng/mL, and 1000 ng/mL) of Cam-β-NGF to obtain a final volume of 1 mL. The treatment groups were kept at 36 °C for 60 min. At 5 min (T0), 30 min, and 60 min of incubation, samples were retrieved to evaluate viability, mass motility, and membrane integrity.
2.6. Statistical Analysis
Results are presented as mean ± standard error of the mean (SEM). GraphPad Prism software version 9.4.0 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses. The D'Agostino & Pearson omnibus test was used to test the normality of data. The differences between semen quality parameters before and after cooling were determined using a non-parametric paired t-test and Mann–Whitney test (p < 0.05). The effects of Cam-β-NGF supplementation were analysed using one-way ANOVA followed by Tukey’s Multiple Comparisons Test (p < 0.05).
3. RESULTS
The effect of short-term conservation (24 h at 4 °C) on mass motility, viability, and membrane integrity is shown in the corresponding Fig. (3A-C).
After 24 h of storage at 4°C, there was a significant decrease in sperm mass motility (5.0 ± 0.0 vs. 2.65 ± 0.41, respectively; p = 0.0048) (Fig. 3A). The viability of preserved sperm was significantly reduced compared to that of fresh sperm before preservation (77.25 ± 3.91% vs. 61.95 ± 10.93%, respectively; p = 0.0032) (Fig. 3B). Furthermore, membrane integrity was significantly decreased after preservation (Fig. 3C), which was expressed as the percentage of swollen spermatozoa (74.90 ± 8.73% vs. 50.67 ± 10.14%, respectively; p = 0.0006).
After supplementation with Cam-β-NGF, samples were then incubated for a total duration of 60 min at 36°C, with measurements taken at T0 (5 min after the start of incubation), T30, and T60 min to assess the effect of each dose on semen quality parameters.
After 5 min of incubation (T0), motility (Fig. 4A), viability (Fig. 4B), and membrane integrity (Fig. 4C) were found to be statistically unchanged.
After 30 min of incubation, the introduction of 1000 ng/mL and 500 ng/mL NGF in the semen extender exhibited the highest mass motility (p < 0.05) in comparison to other concentrations (100 ng/mL and 10 ng/mL) and the control (Fig. 5A). However, viability at T30 (Fig. 5B) did not show a statistically significant difference between the doses and the control. In contrast, membrane integrity (Fig. 5C) was maintained at T30 min with the addition of 1000 ng/mL, 500 ng/mL, and 100 ng/mL NGF in the semen extender compared with the control and extender supplemented with 10 ng/mL NGF (p < 0.05).
After 60 min, the addition of 1000 ng/mL and 500 ng/mL Cam-β-NGF resulted in sustained high levels of mass motility, compared to other dosages and the control (p < 0.05) (Fig. 6A). Moreover, viability remained significantly higher after 60 min of incubation with 1000, 500, and 100 ng/mL, in contrast to the control extender and 10 ng/mL supplementation (p < 0.05) (Fig. 6B). Similarly, membrane integrity was notably maintained with supplementation of 1000 ng/mL, 500 ng/mL, and 100 ng/mL of Cam-β-NGF in the semen extender compared to the control and the lower dose (p < 0.05), leading to a higher proportion of healthy spermatozoa at T60 compared to the control and 10 ng/mL supplemented extender (p < 0.05) (Fig. 6C).
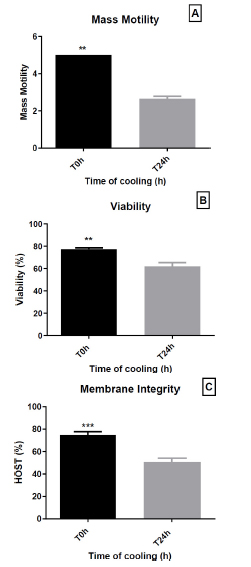
Effect of 24 h cold storage (4 °C) on dromedary sperm quality. (A) Mass motility score; (B) Viability (%); (C) Plasma-membrane integrity assessed by HOST (%). Bars show mean ± SEM (n = 10 ejaculates). T0 h = start of cooling; T24 h = after 24 h at 4 °C. Data are mean ± SEM (n = 10 ejaculates). Statistical significance is indicated by asterisks: ** p < 0.01; *** p < 0.0001.
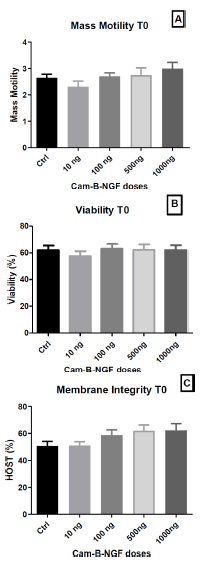
Effect of Cam-β-NGF on dromedary sperm quality at T0 after 24 h of cold storage (4 °C). (A) Mass motility score; (B) Viability (%); (C) Plasma-membrane integrity (HOST, %). Semen was stored for 24 h at 4 °C and then incubated for 5 min at 36 °C (T0) with Cam-β-NGF at 0 (Ctrl), 10, 100, 500, or 1000 ng/mL. Bars show mean ± SEM (n = 10 ejaculates). Different letters denote statistically significant differences among doses (p < 0.05).
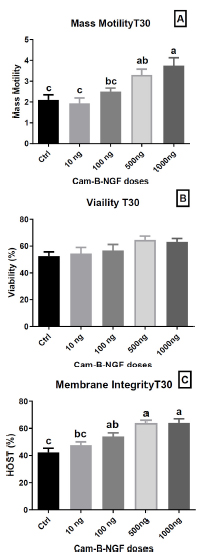
Effect of Cam-β-NGF on dromedary sperm quality at T30 after 24 h of cold storage (4 °C). (A) Mass motility score; (B) Viability (%); (C) Plasma-membrane integrity (HOST, %). Semen was stored for 24 h at 4 °C and then incubated for 30 min at 36 °C (T30) with Cam-β-NGF at 0 (Ctrl), 10, 100, 500, or 1000 ng/mL. Bars show mean ± SEM (n = 10 ejaculates). Different letters denote statistically significant differences among doses (p < 0.05).
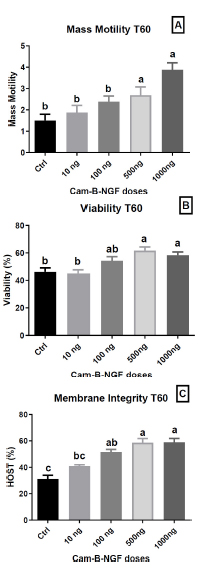
Effect of Cam-β-NGF on dromedary sperm quality at T60 after 24 h of cold storage (4 °C). (A) Mass motility score; (B) Viability (%); (C) Plasma-membrane integrity (HOST, %). Semen was stored for 24 h at 4 °C and then incubated for 60 min at 36 °C (T60) with Cam-β-NGF at 0 (Ctrl), 10, 100, 500, or 1000 ng/mL. Bars show mean ± SEM (n = 10 ejaculates). Different letters denote statistically significant differences among doses (p < 0.05).
4. DISCUSSION
After conservation, spermatozoa lose their traits. In this study, mass motility significantly decreased after 24 hours of storage at 4 °C, and preservation processes affected sperm viability and membrane integrity. This may be due to the elevated levels of unsaturated fatty acids in the sperm plasma membrane and the limited ability of spermatozoa, which contain very little cytoplasm, to fight free radicals. As a result, sperm are highly sensitive to oxidative damage [20, 21].
The plasma membrane of spermatozoa is the primary site affected by low-temperature alterations [22]. Changes in its structure and/or function are often linked to reactive oxygen species (ROS) [23]. Increased ROS production, coupled with a decrease in antioxidants, likely leads to oxidative stress [24], which damages sperm quality and has deleterious effects on spermatozoa. This oxidative damage causes lipid peroxidation, which reduces membrane stability and negatively affects sperm function. Consequently, oxidative stress may explain the decrease in mass motility, viability, and membrane integrity observed in our results. Similar findings have been reported in rats' semen [25-27], ram [28-30], humans [23], alpaca [31-33], llama [34, 35], and camels [36, 37].
In this study, after 24 hours of storage at 4 °C, spermatozoa were treated and subsequently incubated at 36 °C with purified endogenous camel nerve growth factor (NGF) at different concentrations. The goal was to assess whether this protein, which belongs to the neurotrophin family, could help maintain or improve the viability, mass motility, and membrane integrity of camel spermatozoa after storage. The Cam-β-NGF concentrations used in this study (10, 100, 500, and 1000 ng/mL) were selected on the basis of earlier work in camelids, particularly Sari et al. (2021) [34], which reported biological activity of β-NGF within a comparable range.
Cam-β-NGF has been shown to stimulate dromedary camel sperm mass motility in a time- and dose-dependent manner. It is hypothesized that this protein may have receptors on dromedary sperm, potentially enhancing mass motility. These effects are influenced by the presence and distribution of tropomyosin receptor kinase A (TrkA) receptors, which have been identified in the sperm of many species. However, their presence in dromedary camel sperm has not yet been confirmed.
Similar effects have been observed in cooled llama sperm, where supplementation with 10 and 100 ng/mL of exogenous β-NGF improved motility, regardless of incubation duration [34]. In Ilamas, the TrkA receptors were found in the middle piece of the spermatozoon [38], which suggests that β-NGF directly affects sperm motility enhancement in this species. Furthermore, in bulls, these receptors have been identified in the acrosomal cap, nucleus, and tail regions [9]. In addition, Lin et al. [39] reported that NGF could improve the motility of human sperm in vitro by promoting displacement and increasing the proportion of A-grade spermatozoa. Based on these findings in other species, similar mechanisms may occur in dromedary spermatozoa. Immunolocalization studies of Cam-β-NGF receptors (TrkA and/or p75NTR), using techniques, such as immunofluorescence and immunohistochemistry, could provide interesting insights. This research could help develop fertility-enhancing applications in camels.
The addition of Cam-β-NGF maintained sperm viability across all tested doses and the control group at T0 and T30, without significant differences observed. However, after 60 minutes of incubation, sperm viability significantly decreased in the control group and at the 10 ng/mL dose. In contrast, viability remained stable at higher doses (500 and 1000 ng/mL). This result suggests that higher concentrations of Cam-β-NGF may have a protective effect, possibly through its interaction with TrkA receptors. NGF is known to modulate pro-survival factors, preventing cells from entering apoptotic pathways through the activation of the phosphatidylinositide 3-kinase (PI3K) pathway, which plays a crucial role in maintaining cell viability [5]. This pathway is likely involved in sperm survival by inhibiting apoptosis, improving mitochondrial function, and preserving membrane integrity. Sari et al. [34] also reported a positive effect of adding exogenous NGF to cooled llama sperm incubated for 60 minutes with 10 ng/mL. They noted that NGF receptors on sperm are localized in the midpiece, a region directly linked to mitochondrial function [34].
Furthermore, NGF binding to TrkA receptors activates the PI3K pathway, which in turn enhances cell survival [9]. In bulls, Li et al. [9] revealed the presence of NGF and TrkA receptors in ejaculated bovine sperm, and reported that NGF addition (20 ng/L) improved sperm viability after 2 hours of incubation without affecting cellular calcium and acrosome reaction. Although NGF primarily promotes sperm survival through the TrkA receptors, it can also bind to the p75NTR receptor, which may influence apoptosis and necrosis [5]. The balance between these receptor pathways could determine sperm longevity and functionality in the reproductive tract. Further research, including studies with larger sample sizes to overcome the limitations inherent to semen collection in dromedary camels, is needed to elucidate whether the PI3K pathway is directly involved in the NGF-mediated improvements of dromedary sperm viability and to clarify the specific roles of TrkA and p75NTR receptors in sperm survival mechanisms.
Adding Cam-β-NGF improved the sperm membrane integrity of dromedaries in a dose- and time-dependent manner. When sperm are exposed to low temperatures (4 °C), the lipid composition of their membranes can be altered, making them more susceptible to damage and structural instability [40]. This damage affects the membrane’s flexibility and ability to function, which are both important for the sperm’s ability to fertilize an egg. When sperm are stored at low temperatures, it can cause oxidative stress, which overwhelms the sperm’s natural defenses and causes further membrane damage [41]. Interestingly, NGF has been found to help protect the sperm membrane. For example, after thawing bull sperm, those with more NGF mRNA tend to have better membrane quality [42]. This suggests that NGF might reduce the harmful effects of freezing and thawing. Cam-β-NGF could help stabilize the sperm membrane by influencing lipid metabolism and supporting membrane remodeling, which are important for keeping it strong under stressful conditions. In the present experiments, a near-plateau response was detected for most sperm parameters— motility, viability, and membrane integrity— both between 30 and 60 minutes of incubation and between 500 and 1000 ng/mL. To define the full dose- and time-response profile, additional studies with wider concentration intervals and extended incubation periods are required, so that the use of Cam-β-NGF in semen-preservation protocols can be optimized.
CONCLUSION
Our findings suggest that Cam-β-NGF potentially preserves dromedary camel sperm mass motility and viability, and maintains membrane integrity in a time- and dose-dependent manner, likely through a direct interaction with specific receptors on sperm cells. High doses (500 and 1000 ng/mL) showed nearly identical responses, suggesting that testing a broader dose range could pinpoint the true optimum and reveal whether effects decline at higher or lower concentrations. The addition of purified endogenous camel NGF offers a promising approach for the successful short-term preservation of dromedary camel sperm and assisted reproduction techniques. More research is needed to understand exactly how Cam-β-NGF protects sperm and to optimize its use in semen preservation, especially for freezing. Future studies should also examine the effects of Cam-β-NGF on testicular, epididymal, and accessory-gland cells to clarify its role in male-gamete maturation along the entire reproductive tract. Techniques, such as Western blotting or PCR, could help identify receptors and provide further information about their roles.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| β-NGF | = Beta-Nerve Growth Factor |
| Cam-β-NGF | = Camel Beta-Nerve Growth Factor |
| GnRH | = Gonadotropin-Releasing Hormone |
| HOS | = Hypo-Osmotic Solution |
| HBSS | = Hanks' Balanced Salt Solution |
| HEPES | = 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| TCF-EY | = Tris-Citric Acid-Fructose-Egg Yolk Extender |
| FPLC | = Fast Protein Liquid Chromatography |
| PI3K | = Phosphatidylinositide 3-Kinase |
| TrkA | = Tropomyosin Receptor Kinase A |
| p75NTR | = p75 Neurotrophin Receptor |
| SPZ | = Spermatozoa |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All experimental procedures involving animals were approved by the Animal Ethics Committee of the National School of Veterinary Medicine (ENMV), Sidi Thabet, Tunisia (AEC/IACUC). Approval code: CEEA‑ENMV 64/22; approval date: 26 December 2022; internal project reference: 64IRAM2022.
HUMAN AND ANIMAL RIGHTS
This study adheres to internationally accepted standards for animal research, following the 3Rs principle. The ARRIVE guidelines were employed for reporting experiments involving live animals, promoting ethical research practices.
Experimental work complied with the ENMV–Sidi Thabet institutional animal-care and use guidelines and applicable national regulations. Procedures were conducted in accordance with institutional and national guidelines for the care and use of animals in research.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [L.D.], on reasonable request.
FUNDING
The Tunisian Ministry of Higher Education and Scientific Research (Contract program 2020–2023 of LR16IRA04), Tunisia funded this work. The hormonal analysis, the immunofluorescence research, and the Western blotting were funded by CICYT AGL-2017-83799-R and DGA A07 17R.
ACKNOWLEDGEMENTS
The authors would like to thank Belgacem Khchira, Habib Tir, Toumi Secrafi, Gemea Dbouba, Ammar Bahsiss, Houcine Kezaiel, Mohsen Bougueddima, and Mabrouk Fourti for their expertise in semen collection and animal care. Additionally, they are grateful to Dr. Mouldi-Mabrouk Seddik, the veterinarian, for his support in monitoring and ensuring the health and well-being of the animals during the study.


