All published articles of this journal are available on ScienceDirect.
Evaluation of the Bioactivity of Botryosphaeria Species Isolated from Acacia Species
Abstract
Introduction
Current research emphasizes the endophytic fungi that produce secondary metabolites for biotechnological relevance. Botryosphaeriaceae species can produce significant bioactive compounds, and the Botryosphaeria genus is gaining attention in this regard.
Methods
Botryosphaeriaceae species were isolated from Acacia karroo. Morphological and molecular identification was conducted using ITS and BOT primers. The dual method was utilised to determine the antifungal properties of the endophytic fungi. The PCR amplicons were sent for sequencing.
Results
The overall colonization rate reached 74%, with 18% attributed to the target species. Most isolates exhibited rapid growth, except for isolate 68. Nine isolates were identified as members of the Botryosphaeriaceae family via amplification with BOT primers. Isolate 13 showed strong antifungal effects against two test pathogens, while isolate 78 displayed moderate activity. BOT primer amplification yielded a 372 bp product. BLAST analysis identified isolate 13 as Botryosphaeria dothidea (MT197291.1; 92.76% identity) and isolate 78 as B. dothidea (AF027746.1; 98.41% identity). Endophytic fungi inhabit plant tissues asymptomatically, often engaging in mutualistic associations.
Discussion
This study confirms the high colonization of endophytic fungi and identifies Botryosphaeria dothidea with notable antifungal potential. Isolate 13 showed strong inhibition against test pathogens, suggesting possible biocontrol applications. Molecular and morphological tools effectively confirmed species identity. Limitations include the use of a single primer and limited pathogen screening.
Conclusion
The endophytic fungi isolated from Acacia karroo have the potential to be used as fungicides in the agricultural sector. These bioactive compounds have enormous prospects in the biotechnological sphere.
1. INTRODUCTION
Several studies have identified novel bioactive secondary compounds isolated from endophytic fungi that could be utilized in drug development [1-8]. It is common in nature for two different species to coexist in a symbiotic relationship, and a typical example is the symbiotic relationship between plants and fungi. Endophytic fungi live inside the host plant tissues without causing any damage or disease symptoms [9-11]. The endophytic microbe is provided with nutrients and shelter, whilst the host can tolerate both biotic and abiotic stress [12]. Moreover, endophytic fungi have been reported to produce bioactive metabolites that might be used for industrial and pharmaceutical applications [11]. A study conducted by Jami et al. [13] has reported that Botryosphaeriaceae species were found on four unrelated hosts, hence highlighting their ability to infect a wide range of native plant species in South Africa. In this work, V. karroo will be referred to as Acacia karroo for the reasons stated by Alfred Maroyi [14]. A. karroo is identified as a brown to near-black tree with pairs of projecting thorns. Kurt et al. [15] have recently isolated Botryosphaeriaceae species from citrus plants in the eastern Mediterranean region of Türkiye.
The Botryosphaeriaceae family belongs to species characterized by fast growth, white to black mycelia, and aerial hyphae on a culture medium [16]. These species are reported to infect A. karroo without, at times, showing symptoms [17], thereby making it difficult to identify them from the host species. The Botryosphaeria genus is one of the easiest to isolate, with Botryosphaeria dothidea being the most abundant of the isolates. B. dothidea infects pears, apples, apricots, and most woody plants [16, 18]. B. dothidea can infect A. karroo with no tissue specificity, infecting both branches and leaves through wounds and natural openings [13]. B. dothidea can produce secondary metabolites of pharmaceutical interest [11]. Valente et al. [11] highlighted the successful effects of B. dothidea secondary metabolites as immunosuppressants and antioxidants. Further stated that the advantage of bioactive compounds as compared to synthetic compounds is that they may result in toxic compounds during manufacture and within formulation.
There are limited results reported on the bioactive properties of B. dothidea, which sparks interest in exploring bioactive compounds produced by this species isolated from Acacia species as a potential novel source. Botryosphaeria spp. have been reported to have an endophytic phase and the ability to produce bioactive compounds. However, there is limited literature covering the production of bioactive compounds by Botryosphaeria spp. This work aimed to isolate B. dothidea and other Botryosphaeriaceae species from A. karroo and assess their antimicrobial properties.
2. MATERIALS AND METHODS
2.1. Collection of Plant Samples
The samples were collected from the Mmabatho area (25°50’25” S; 25°36’31” E), North West province in South Africa. Twenty-five samples of Acacia karroo were collected from five different locations within Mafikeng. All samples collected were disease-free, without symptoms of pathogen infection. The samples were covered with newspaper to reduce transpiration rates. The samples were stored in a cold room (at 4°C) and processed within a week of collection. Two more branches were pressed using a plant press, and the specimens were stored/preserved in the herbarium.
2.2. Isolation of Fungal Species
The leaflets were surface-disinfected by immersion in 70% (v/v) ethanol for 60 seconds and then rinsed in distilled sterile water (dsH2O) for 60 seconds, a modification [15, 16]. The leaflets were subsequently plated on Potato Dextrose Agar (PDA; Merck-Biolab, Gauteng, South Africa), five leaflets per plate, totalling 100 PDA plates. The plates were incubated at room temperature until visible growth occurred. The colonization frequency was then determined using the following formula (1) [16]:
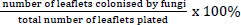 |
(1) |
2.3. Morphological Identification of Fungal Species
Preliminary identification of Botryosphaeriaceae was based on the morphological features of the fungal colony. Pure isolates were prepared by subculturing on fresh 65mm diameter PDA (Merck-Biolab, Gauteng, South Africa) plates fortified with kanamycin sulphate (VWR Life Science, England) and chloramphenicol (VWR Life Science, England). The macroscopic identification included observation of colony colour from both the front and the bottom of the plates. Microscopic identification was performed using a Reichert-Jung model 150 microscope (Cambridge Instruments Inc., New York, United States of America). On day three of the pure colonies, hyphal tips were grown on 2% (w/v) Agar bacteriological (Merck-Biolab, Gauteng, South Africa) to induce sporulation, which was then autoclaved (Sturdy SA-300VL; Sturdy Industrial Co., Ltd., Taiwan). Acacia karroo leaflets were used as a substrate. The plates were incubated (Labcon FSVE-SP08, Labcon, South Africa) at 25°C for at least four weeks or until sporulation was achieved. Isolates from Agar bacteriological plates were observed under a microscope (Cambridge Instruments Inc., New York, United States of America) to note the morphology of the spores and hyphae, estimate the length-to-width ratio, and provide general descriptions. Special structures were also noted where present.
2.4. Growth Rate
The pure fungal isolates were grown on PDA (Merck-Biolab, Gauteng, South Africa). The growth diameters were monitored for the seventh day. The first and the last days were used to calculate the growth rates.
2.5. Molecular Identification of Fungal Species
DNA was extracted using the Zymo Research Quick-DNATM Fungal/Bacterial Miniprep Kit (Catalogue number D6005), supplied by Inqaba Biotec (Pretoria, South Africa), according to the manufacturer’s instructions. Primers ITS1 and ITS4 (Table 1) were used for amplification of the internal transcribed spacer regions (ITS) of ribosomal DNA (rDNA), as described in previous studies [10, 13]. The thermocycler conditions were set to 95 °C for 5 minutes for initial denaturation, followed by 30 cycles of 94 °C for 30 seconds, 58 °C for 30 seconds and 72 °C for 60 seconds for denaturation, primer annealing and elongation, respectively and final elongation set at 72 °C for ten minutes [19]. In addition, primers BOT100F and BOT472R (Table 1) were used to detect and amplify Botryosphaeriaceae species by amplifying a 371-372 bp region of the rDNA [19, 20]. The thermocycler conditions were similar to those of ITS1 and ITS4.
| Primer1 | Sequence |
|---|---|
| ITS1 | 5’-TCCGTAGGTGAACCTGCGG-3’ |
| ITS4 | 5’-TCCTCCGCTTATTGATATGC-3’ |
| BOT100F | 5’-AAACTCCAGTCAGTRAAC-3’ |
| BOT427R | 5’-TCCGAGGTCAMCCTTGAG-3’ |
The PCR mixtures had a total volume of 25 µL for all primers. They were made up by 12.5 µL One Taq® Quick-load® 2x Master mix with standard buffer (Inqaba Biotec, Pretoria, South Africa), 0.5 µM each of the reverse and forward primers, 1 µL of DNA template, and filled to the final volume using nuclease-free water (Inqaba Biotec, Pretoria, South Africa). Amplifications were performed using a Bio-Rad C1000 Touch™ Thermal Cycler (Lasec, Johannesburg, South Africa). Five (5) µL PCR amplicons were used for gel electrophoresis. The PCR products were separated using a 1% (w/v) agarose gel stained with ethidium bromide at 10 V·cm-1 for 50 minutes and visualized by using a Spectroline UV Transilluminator SlimlineTM series, model TE-312S/F (Lasec, Johannesburg, South Africa). The fungal isolates that showed strong antifungal activity were selected, and their PCR amplicons were sent to Inqaba Biotech (Pretoria, South Africa) for sequencing. The sequences were cleaned, aligned, and consensus sequences were constructed. The sequences were analyzed using BLAST via the NCBI Web Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to confirm the fungal identities.
2.6. Antifungal Activity
Antifungal activity was evaluated against four agriculturally important fungal pathogens. The evaluation was carried out using the dual method, which was a plate confrontation method. A small quantity of mycelium of both the pathogen and the isolate was inoculated at the opposite edges of PDA plates (Merck-Biolab, Gauteng, South Africa) using a sterile needle (25 ± 2 °C for 7 days). The colony growth of the fungal isolates was measured. The fungal pathogens were Fusarium graminearum, Colletotrichum gloeosporioides, Fusarium sp., and Aspergillus sp. A control group was included for each pathogen, where the pathogen was inoculated alone under the same conditions, to assess its uninhibited growth. The percentage inhibition of the growth of the pathogen was calculated with the help of the formula (2):
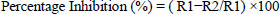 |
(2) |
Where:
- R1 = radial growth of the pathogen in the control plate (mm)
- R2 = radial growth of the pathogen towards the antagonist in the dual culture plate (mm)
2.7. Statistical Analysis
The activity of the culture filtrate was calculated using the formula given μ=(μ_1+μ_2)/N, where μ= mean, μ (1 and 2) = the measured diameter on each plate, and N = the number of plates representing a particular isolate. The confidence level is 95%, and the data handling is used to assess significance (p < 0.05). Data were presented as means with standard deviations, and all experiments were performed in triplicate to ensure reliability.
3. RESULTS AND DISCUSSIONS
From the 500 leaves initially plated, 90 isolates showed cultural growth characteristic of Botryosphaeriaceae species. Pure colonies were subcultured from the 90 presumptive isolates, and only 29 culture plates were selected as presumptive Botryosphaeriaceae species. Fig. (1) below presents the colonisation frequencies of overall, presumptive (after subculturing), and isolates closely related to Botryosphaeriaceae.
The low colonization frequency (5.8%) observed in this study is similar to the results obtained by [13] in 2008. However, their results showed an increase to 25% in the following two years. These results were obtained from a comprehensive survey of Botryosphaeriaceae species isolated from Acacia karroo in South Africa [13]. The results further revealed a less diverse collection of Botryosphaeriaceae species, with only Botryosphaeria dothidea and Spencermartinsia viticola isolated in the North-West [13], where this current study was conducted. A relationship also exists between the time of sampling, the host tissue sampled, and species diversity [13]. Jami et al. [13] also reported that some species have seasonal occurrence, thus occurring in certain years and being absent in others. They further reported higher species diversity in leaves.
Furthermore, the very same physical barriers that protect the leaves from microbial pathogens could have played a part in hindering the exit of endophytes and growth on the media, since whole leaflets instead of excised portions were plated. The related species were divided into seven groups based on the cultural growth similarities after four weeks (except group 7) without considering the growth rate of the isolates. The resulting groups are presented in Table 2. Group 7 had only two isolates whose characteristics did not match those of other groups, and between them.
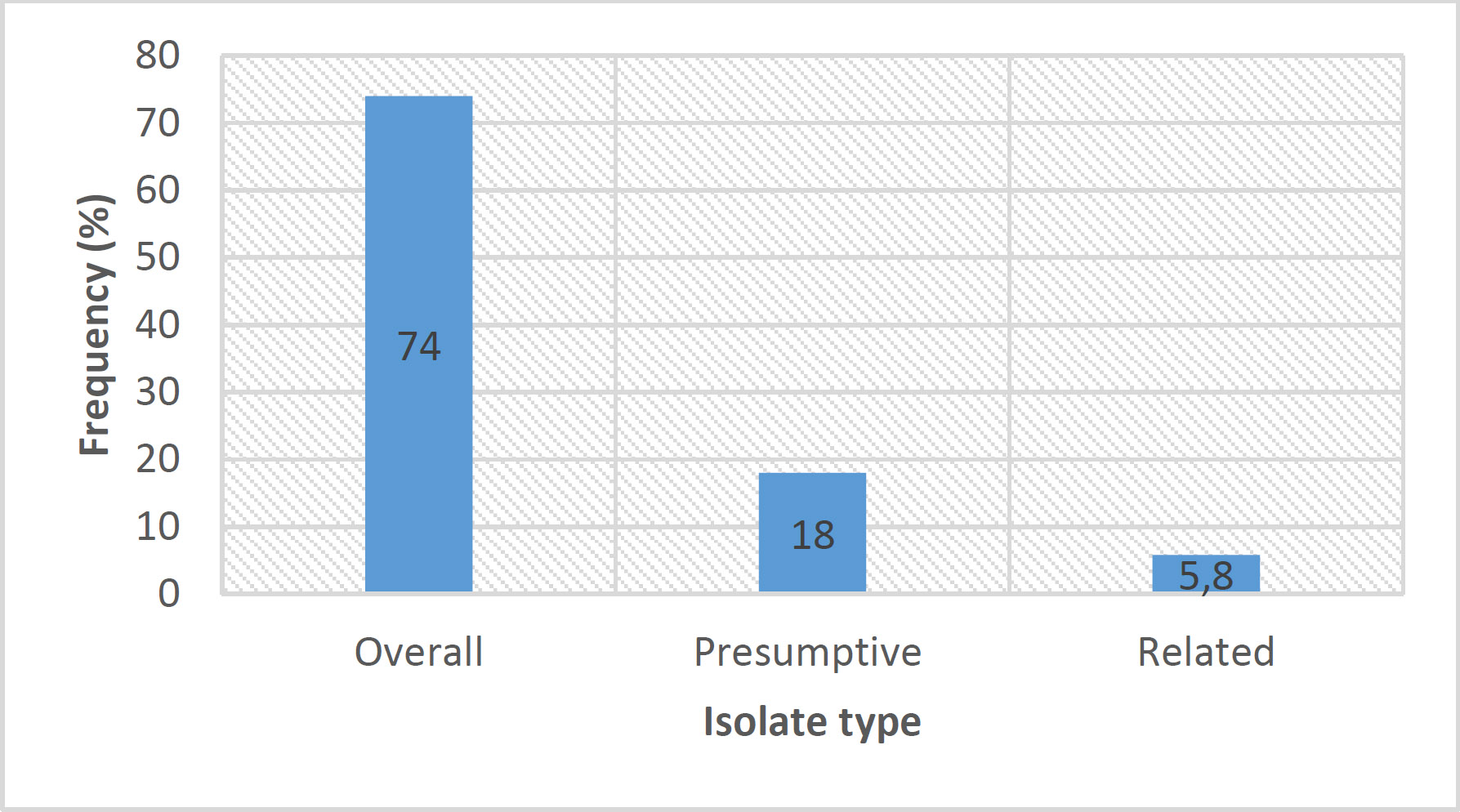
Colonisation frequencies of the isolates. The numbers within the bars represent the frequency percentages.
| Group Number | Isolate ID Number |
|---|---|
| 1 | 23; 78; 80; 82; 84 |
| 2 | 5; 19; 31 |
| 3 | 13; 28; 37; 41; 44; 53; 60 |
| 4 | 15; 16; 22 |
| 5 | 9; 10; 20; 74; 75; 83 |
| 6 | 14; 42; 68 |
| 7 | 32; 65 |
The grouping of the isolates is explained above. Generally, the isolates were initially white, with the development of pigmentation starting on day 3. All isolates were aerial and touched the lid except isolates 65, isolate 5, group 4, and group 5. In group 4, isolate 16 was the only one that felt the lid. Table 3 summarises observed morphological traits on days 3, 5, and 7. The characteristics described in Fig. (2) fit the description of Botryosphaeria species, including B. dothidea as defined by [21]. Though macroscopic characteristics were used to select Botryosphaeriaceae species, they were rather unreliable when determining the genus and species epithet. This is due to overlaps of these characteristics between genera and within a specific genus [22]. Therefore, microscopic traits were considered to increase the confidence level.
All 29 isolates had hyaline and septate hyphae. All the genera were identified using the keys provided in the study by Phillips et al. [21]. Isolates 13, 14, 15, 16, 23, 31, 78, 80, and 82 had conidia that were either hyaline or brown. The observed conidia were aseptate without striations. No germ slit, pycnidial paraphyses, or mucous sheath observed. Based on these traits, these species belong to the Diplodia genus. This cluster, therefore, represents 31% of the isolates that resembled Botryosphaeriaceae on PDA. Another group was comprised of isolates 5, 28, 32, 37, 42, 53, 65, 68, 74, and 83. These isolates produced hyaline conidia that were thin-walled and more or less fusoid, sometimes ellipsoidal. The conidia had no mucous sheath. These species were identified as Botryosphaeria or Neofusicoccum as per Phillips et al. [21]. However, conidial morphology and pigmentation assured the species fell under the Botryosphaeria genus. Additionally, isolates 5, 32, 37, 53, 65, and 74 had a weight ratio (l/w ratio) that was greater than 4.5.
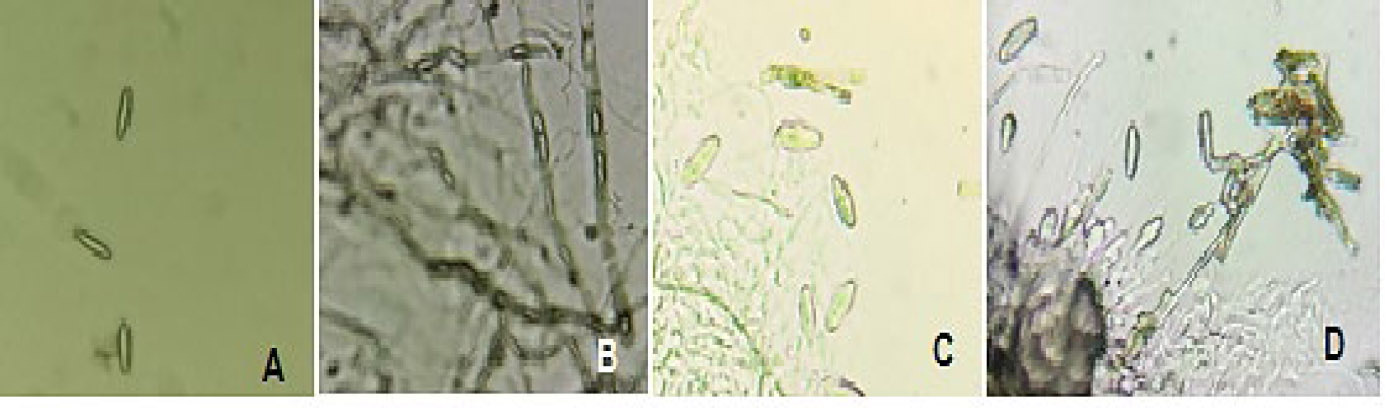
Microscopic pictures of the isolates. Pictures A, B, C and D belong to isolates 5, 19, 32, and 65, respectively. The images are not presented to scale.
| Group Number | Day 3 | Day 5 | Day 7 | Special Notes |
|---|---|---|---|---|
| 1 | • Grey pigmentation developing from the centre. | • Colonies were grey to light grey. | • Colonies got darker. • Isolate 82 had green-grey pigment that was observable from the bottom. |
• On the seventh day, isolates 80, 82 and 84 were smokey-grey1, as observed from the top. |
| 2 | • Isolates had green-grey colouration. | • Colonies were grey. | • Colonies were almost black. • Colonies had filled the plates. • Isolate 19 was developing small, spherical fruiting bodies |
• The fruiting bodies were initially about 1 mm in diameter and turned black over time, producing a clear, viscous fluid. |
| 3 | • Isolates were turning grey. • Isolate 13 had green-grey pigmentation. |
• Isolates progressed to dark grey and black. | • Isolates had filled the plates. | • Isolate 53 had irregular edges. |
| 4 | • Isolates had green grey pigmentation that developed from the center (except isolate 16). • Isolate 16 was white and touching the lid. |
• Isolates progressed to dark grey and black. • Isolate 16 was as others on day 3. |
• Isolates 15 and 22 had remained dark grey to black with the development of small, white spherical fruiting bodies about 1 mm in diameter, as observed in 19. | • Fruiting bodies had turned black in the fourth week of growth. • Isolate 16 showed delayed development and turned grey on day 7 |
| 5 | • Isolates had green grey pigmentation that developed from the center (except isolate 83). | • Isolates 9, 10, and 20 had dark grey to black bottoms and smoky grey tops. • The rest of the isolates were grey. |
• Isolates 9, 10, and 20 had grey tops and dark grey to black bottoms. • The rest of the isolates had colours reported for isolate 10 on day 5. • Isolates 20, 75, and 83 started developing fruiting bodies as observed in isolate 19. |
• The fruiting bodies were arranged in clusters and formed a ring with slight green coloration. • The fruiting bodies turned black and bigger in the fourth week. |
| 6 | • Isolate 42 had a grey centre, whereas the rest were white. | • No changes observed. | • All isolates had a green-grey centre. | - |
| 7 | • Isolate 32 was touching the lid. • Isolate 65 had a light grey centre observable from the bottom. |
• Isolate 32 progressed to grey and had irregular edges. • Isolate 65 had no significant changes |
• No further changes were observed on isolate 32. • Isolate 65 had a grey centre with white edges observable from the bottom and top of the plates. |
- |
This further indicated that these were B. dothidea species. This group represents 34.5% of the isolates. Representative microscopic images were taken and presented in Fig. (2A-D). The last group included isolates 9, 10, 20, and 60, which exhibited terminal club-like structures. Isolate 19 had hyaline, spore-sized structures within its hyphae. The spore-like structures were cylindrical to subobtuse at one end. Isolates 22, 75, and 84 had more or less clavate spores that were striated or divided into blocks. Lastly, isolates 41 and 44 produced brown chlamydospores. Due to a lack of similarity to Botryosphaeriaceae species, these were ruled out for a possibility of being a Botryosphaeriaceae species.
Fig. (3A-G) below presents a graphical representation of the growth rates of the investigated isolates. The growth rates were determined on day seven. Isolates with growth rates between 0 and 2.75 mm/day were classified as slow-growing fungi, growth rates between 2.76 and 5.51 mm/day and 5.52 and 8.25 mm/day were categorized as moderate and fast-growing fungi, respectively. Subsequently, based on the aforementioned criterion, all isolates were fast-growing except isolate 68, which had a moderate growth rate. Since Botryosphaeriaceae species are fast-growing species [16], the isolates, except isolate 68, fit another trait as presumptive isolates.
DNA extraction was successful for all isolates. Figs. (4 and 5) below represent the amplicons of ITS (ITS1 and ITS4) and BOT (BOT100F and BOT427R) primers on a 1% agarose gel, respectively. All isolates amplified with the ITS primers (Fig. 4), indicating that the DNA would also amplify when using BOT primers, but only on target species. This is substantiated by Figs. (4 and 5). All the ITS amplicons had bands between 500 and 750 base pairs (bp), but closer to the 500 bp mark than the 750 bp band, which sets the estimated amplicon size to approximately 550 bp. This is close to the expected size of the amplicons [18]. Morphological identification was based on colony and spore characteristics using standard taxonomic keys. Molecular identification using BOT primers targeted the β-tubulin gene, which provides higher resolution for species-level classification. The results from both methods were consistent, with molecular data confirming most morphological identifications.
A total of nine (n = 9) isolates were confirmed to be of the Botryosphaeriaceae using BOT primers; isolates 5, 9, 19, 20, 22, and 31 were negative. These amplicons could not be optimised since Tennakoon et al. [23] reported the propensity of the primers to amplify non-target species at a different temperature. Nonetheless, the positive isolates had amplicons centred between 250 and 500 bp, which was estimated to be around 375 bp. The size reported and expected for positive amplicons is 372 bp [20, 23], which is approximately the same size as obtained in this work. The positives (including isolates 13, 15, 15, and 82 that produced faint bands) can be said with certainty to be Botryosphaeriaceae species.
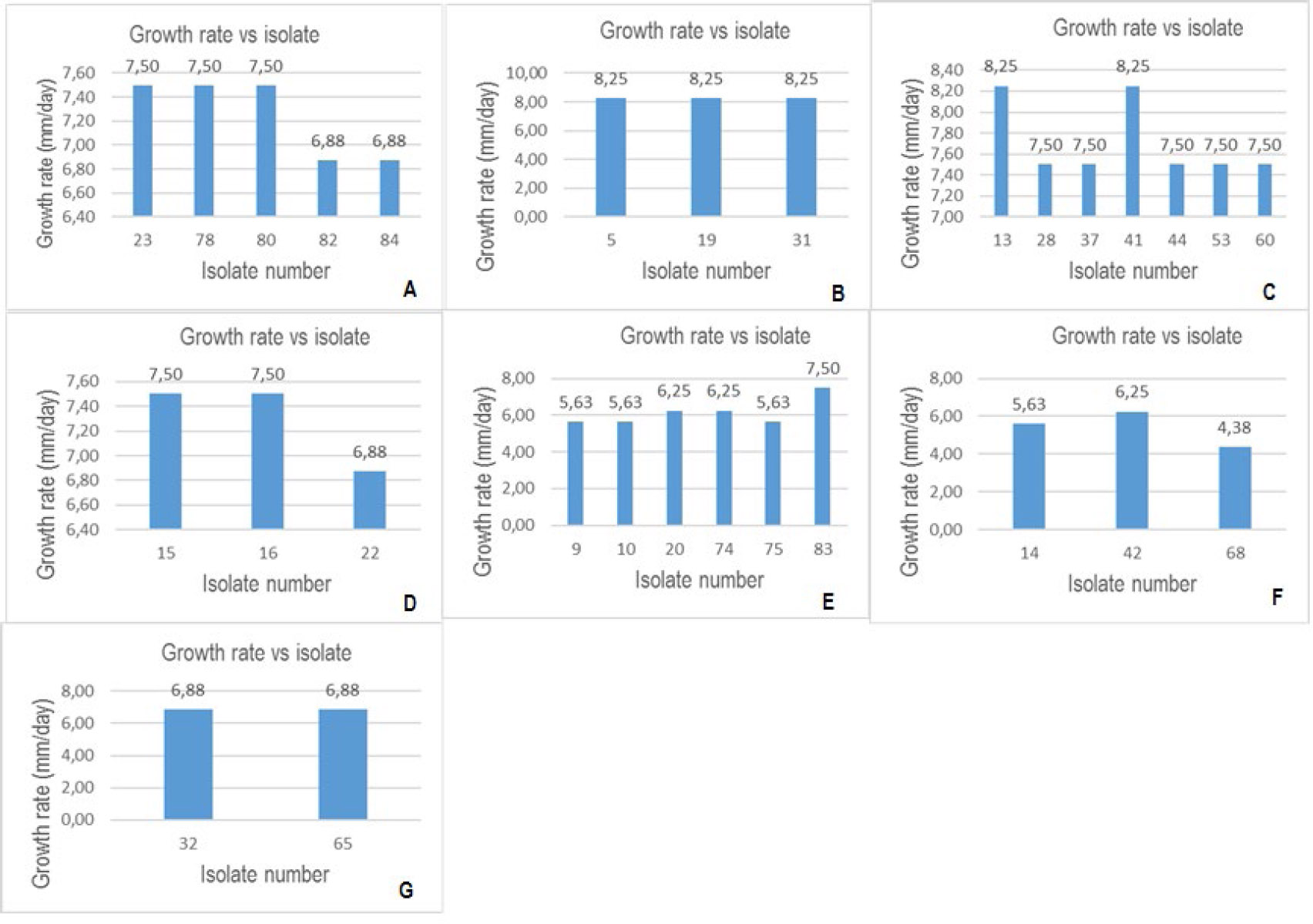
Growth rates of the isolates. Graphs A to G represent growth rates for isolates in groups 1 to 7, respectively.
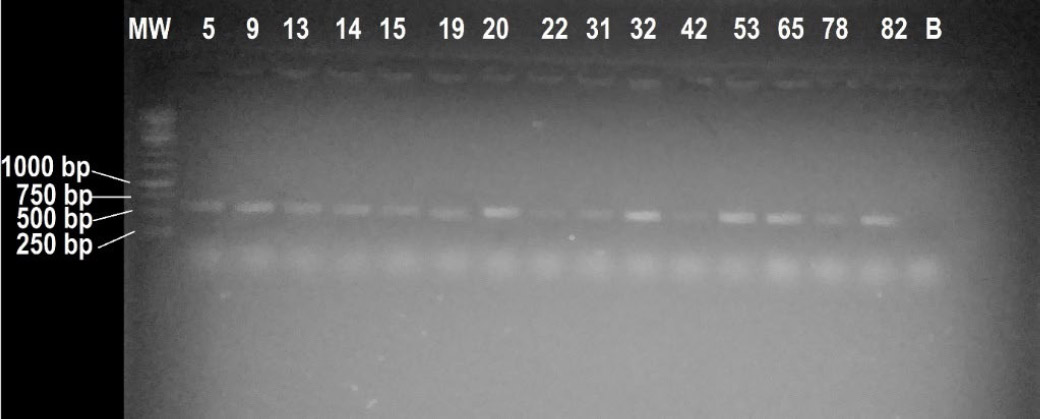
Visual presentation of the ITS amplicons of the isolates. MW represents a molecular marker, and the rest of the numbers on top represent the lanes in which the respective isolate amplicon was loaded. Lane B corresponds to a blank; the numbers on the left show the size of the bands where the lines are pointed.
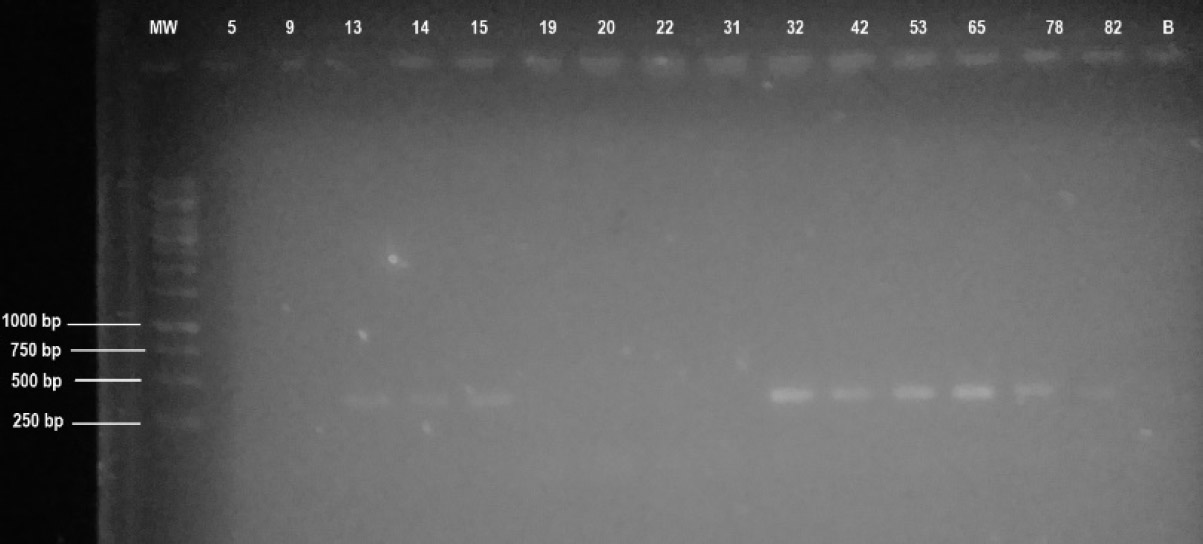
Visual presentation of the BOT amplicons of the isolates. MW represents a molecular marker, and the rest of the numbers on top represent the lanes in which the respective isolate amplicon was loaded. Lane B corresponds to a blank. The numbers on the left show the size of the bands where the lines are pointed.
| Fungal Pathogen1 | Fungal Isolates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 13 | 14 | 15 | 31 | 32 | 42 | 53 | 65 | 78 | 82 | |
| F. graminearum | - | - | - | - | N | - | - | - | - | - | - |
| Aspergillus sp | - | - | - | - | - | - | - | - | - | - | - |
| Fusarium sp | + | +++ | - | - | N | + | N | - | + | - | - |
| Colletotrichum gloeosporioides | + | + | + | + | - | N | + | - | + | ++ | - |
Although isolate 5 was negative, microscopic morphology suggests that it is likely Botryosphaeria dothidea. BOT primers do not amplify B. dothidea and B. eucalyptorum due to high sequence variability [23].
The antifungal activity results are summarised in Table 4. Isolates 9, 20, 19, and 22 were not assessed since they were determined to be non-Botryosphaeriaceae species. Among the four pathogens tested, Colletotrichum gloeosporioides is relatively the weakest pathogen that is susceptible to all isolates except isolates 31, 53 and 82. These isolates did not exhibit any antifungal activity. Liu et al. [24] isolated two new tetraketide-derived phenol rhamnosides and a new rhamnosylated tryptophol alkaloid from endophytic Botryosphaeria dothidea LE-07. The rest of the isolates exhibited activity against at least one of the pathogens; however, isolate 13 stood out with the highest antifungal activity against Fusarium spp., followed by isolate 78, which showed moderate activity against Colletotrichum gloeosporioides. Thus, this present study revealed that endophytes have the potential to produce bioactive compounds with significant antifungal activity, as reported in [25].
CONCLUSION AND FUTURE PROSPECTS
It is well known that all plant species harbor endophytes; however, specific endophytes are adapted to inhabit specific hosts rather than associating with a random host. Dual culture assays demonstrated significant inhibition against selected pathogens. However, the limited sample size may affect the generalizability of the findings. Other limitations include environmental variability and culture-dependent methods, which may not detect all fungal species. Additionally, seasonal and geographical constraints could affect the generalizability of the results. The sample size was determined based on the availability of representative samples, resource constraints, and the need to ensure statistical reliability while maintaining a manageable data collection and analysis process. It provides sufficient power to detect meaningful differences or patterns relevant to the study objectives. The current study showed that Botryosphaeriaceae, particularly Botryosphaeria dothidea, may produce bioactive compounds exhibiting strong antifungal activity against agricultural pathogens.
Additionally, the bioactive compounds responsible for the antifungal activity were not purified or chemically characterized in this study. Moreover, future studies will focus on the purification and identification of the active compound. This will allow for a better understanding of the specific bioactive constituents responsible for the observed antifungal effects. Additionally, characterization of these compounds could pave the way for their development into potential biocontrol agents or pharmaceutical leads. In agriculture, Botryosphaeria is studied for its role as a pathogen causing diseases like black rot in fruit crops. Its ability to decompose organic matter aids in improving soil health and enhancing nutrient cycling. Additionally, certain strains are explored for biocontrol applications to manage other plant pathogens, offering a sustainable alternative to chemical pesticides. In conclusion, the present study endorses the utilization of endophytic Botryosphaeriaceae as a source of novel bioactive molecules for biotechnological advancements.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: R.N.: Conducted the experiments, performed data analysis, and drafted the manuscript; C.N.A. and M.C.M.: Supervised the research, contributed to the conceptualization, and provided input on data interpretation, manuscript review, and editing. All authors read and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ITS | = Internal Transcribed Spacer Regions |
| rDNA | = ribosomal DNA |
| PDA | = Potato Dextrose Agar |
| BP | = Base Pairs |
| DSH2O | = Distilled Sterile Water |
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the North-West University Institutional Repository at https://repository.nwu.ac.za/home.
ACKNOWLEDGEMENTS
The authors would like to acknowledge their respective universities and departments for supporting this study.


