All published articles of this journal are available on ScienceDirect.
Effect of the Combinatorial use of Naringin and Silver Nanoparticles on Enterococcus Faecalis
Abstract
Introduction
This study aims to evaluate the antibacterial activity of naringin and its aglycone derivatives, alone and combined with silver nanoparticles (AgNPs), against E. faecalis, focusing on bacterial morphology and virulence gene expression.
Methods
The antimicrobial activities of naringin and its derivatives were evaluated through the minimal inhibitory concentration (MIC), minimal bactericidal concentration (MBC), and checkerboard assays, while an ex vivo human tooth model tested irrigation efficacy. Gene expression was analyzed via qPCR, and bacterial morphology changes were examined using scanning electron microscopy.
Results
Naringin and its derivatives inhibited 50% of E. faecalis growth at concentrations above 21,500 μg/mL. Combining naringin with AgNPs did not boost antimicrobial activity but disrupted typical bacterial pairing and increased bacterial aggregation. Notably, this is the first study to report that antimicrobial concentrations of naringin and its derivatives upregulate genes associated with stress protection and biofilm formation in E. faecalis.
Discussion
The idea of combining natural flavonoids with AgNPs for infection control during endodontic procedures is promising for reducing tissue damage. However, the findings suggest that simply mixing these compounds does not guarantee better antibacterial effects and may even hinder them. Study Limitations include difficulties in evaluating biofilm-specific effects and solubility challenges with naringin, highlighting the need for further optimization.
Conclusion
Naringin may increase E. faecalis tolerance to stress, weakening the antibacterial impact of AgNPs when combined. Its role in biofilm development requires more research to understand and harness its potential in dental antimicrobial strategies fully.
1. INTRODUCTION
Enterococcus faecalis (E. faecalis) is a Gram-positive, facultative anaerobic bacterium commonly found in the gastrointestinal tract but reported as an opportunistic pathogen that can cause serious infections in humans and is firmly established as one of the major nosocomial pathogens. The oral cavity has been frequently associated with root canal infections, primary endodontic infections, and failed endodontic treatments [1]. This is due to its resistance to antiseptics and disinfectants [1], its ability to survive harsh conditions [2], and multiple antibiotic resistance [3].
Studies on irrigation solutions in dentistry have evaluated and demonstrated significant antimicrobial efficacy and recommend, by current guidelines, the disinfection of the root canal with ideal agents, such as sodium hypochlorite and chlorhexidine gluconate. However, these commonly used irrigation solutions reduce cell viability and survival of dental pulp and even lead to the formation of para-chloroaniline-like precipitates with cytotoxic and carcinogenic effects [4, 5]. Considering these unwanted effects, the rise of microbial resistance to antibiotics, and a growing number of multidrug-resistant pathogens [6], biomedical science has focused significantly on discovering, developing, and reengineering new therapeutic approaches, especially natural products. Substantial research provides evidence that natural molecules, including bioflavonoids, exhibit antioxidant, anti-inflammatory, anticancer, and antimicrobial activities [7–10].
Research has demonstrated that bioflavonoids exhibit antimicrobial activity against a wide range of microorganisms, including those associated with oral diseases [11–13]. Bioflavonoids exhibit a wide range of biological and pharmacological properties, and their potential as endodontic disinfectants has recently been evaluated in solution-based applications. For example, quercetin has shown bactericidal effects against E. faecalis biofilms without cytotoxic effects, making it ideal for use as a reliable adjunctive irrigant solution [14]. Among the most investigated bioflavonoids is naringin, which occurs naturally in citrus fruits, tomatoes, grapes, roots, and various vegetables [15]. Naringin, a hydrogenated glycoside belonging to the flavanone category, has two aglycon forms: naringenin and prunin. Through enzymatic modification by naringinase, naringin can be deglycosylated to produce naringenin and prunin, which enhances the absorption and biological properties of these flavanones [16]. Naringin has not been assessed into irrigant solutions, and it may exhibit appropriate characteristics as an endodontic solution.
Emerging nanotechnology has developed various carbon or metal nanoparticles, providing another therapeutic approach against important microbial agents [17]. Silver nanoparticles (AgNPs) have exhibited strong antimicrobial activity against several microorganisms, and they have been used in different areas of dentistry for disinfection, prophylaxis, and prevention of infections [18]. The combination of AgNPs with plant extracts’ phytochemicals has been previously explored, demonstrating enhanced therapeutic effectiveness [19], and flavonoid encapsulation in nanoparticles has shown a positive effect on the regulation of pro-inflammatory cytokine production [20]. A recent comprehensive review highlighted the biological properties of a wide range of flavonoid–silver nanoparticle (AgNP) conjugates and hybrids, demonstrating their potent anti-inflammatory and antimicrobial activities [21]. Therefore, the study aimed to evaluate the anti-E. faecalis activity of naringin and its aglycone derivatives obtained by enzymatic hydrolysis of prunin and naringenin, both individually and in combination with silver nanoparticles. This was explored as an alternative method to prevent E. faecalis root canal infection. This study investigated the effects of exposure to these individual and combinatorial compounds on bacterial growth, morphology, and changes in virulence-associated genes.
2. MATERIALS AND METHODS
2.1. E. faecalis, Preparation of the Media, and Growth Conditions
E. faecalis ATCC 29212 was cultured in blood agar and incubated at 37ºC and then sub-cultured in Mueller-Hinton agar (Scharlau 01-136). For all experiments, E. faecalis was suspended and grown in Brain Heart Infusion (BHI) broth, adjusting the bacterial suspension to a density of 3x108 CFU/ml (OD600) corresponding to the 1 McFarland scale.
2.2. Preparation of Naringin and Hydrolysed Naringin
Naringin (Sigma-Aldrich, N1376) dissolution was made with 10% dimethylsulfoxide (DMSO). To obtain citrus naringin derivatives with better functional properties, such as prunin and naringenin, we performed the deglycosylation of naringin by its hydrolysation with naringinase (Sigma-Aldrich, N-1385, Lot: 042K1613).
The enzymatic hydrolysis of naringin 0.5% (50 mL) by naringinase was performed in a jacketed cylindrical glass reactor (internal diameter of 3 cm, height of 6 cm) operating in a batch regime. The reaction was conducted at 60 °C with constant agitation at 400 rpm, using a naringinase concentration of 1 mg/mL at 5% (v/v). Samples (0.25 mL) were collected over time from the unmodified naringin solution. After 8 hours, the reaction yielded a derivative compound referred to as hydrolyzed naringin (NH), which contained prunin, naringenin, and residual sugars such as rhamnose and glucose. Naringinase activity was determined using spectrophotometric methods: flavone quantification by the Davis method [22] (ε = 53.888 M−1 cm−1) and reducing sugar analysis by the DNS method [23] (ε = 91.246 M−1 cm−1). The final product was then lyophilized.
2.3. Preparation of AgNPs
Silver nitrate (AgNO3) CAS-N° 7761-88-8 from Merck, sodium borohydride (NaBH4) CAS-N° 16940-66-2 was purchased from AppliChem GmbH, sodium citrate dihydrate (Na3C6H5O7*2H2O) CAS-N° 6132-04-3, was procured from Sigma Aldrich. All chemicals used were analytical grade and were utilised without further purification, and deionized ultrapure water was used for the dilution of chemicals throughout the study.
For the synthesis of AgNps, a solution was prepared using 125 mL of distilled water, which contained 15.7482 mg of AgNO3 and 72.0825 mg of Na3C6H5O7*2H2O. This CIT-doped AgNO3 solution is added dropwise to a previously prepared solution of 8.7682 mg of NaBH4 in 125 mL of distilled water. The entire system is placed under magnetic stirring and controlled at a temperature of approximately 4°C using water and ice. Before starting the synthesis, both solutions were homogenised using the indirect ultrasonic treatment for 5 minutes and then placed in a freezer for an additional 5 minutes at -18°C.
Once the silver nanoparticles were synthesized, the quantification of AgNPs in the samples was verified by inductively coupled plasma (ICP) analysis and graphite furnace atomic absorption spectroscopy (GFAAS). Structural and morphological analysis was performed by dynamic light scattering (DLS) and X-ray diffraction (XRD) analysis.
2.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Determinations
This study followed the guidelines of the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS, 1993). We assessed qualitative MIC values visually for the absence of turbidity, following the Wiegand et al. protocol [24]. Additionally, we determined quantitative MIC using the microdilution method. Enterococcus faecalis from the stock colony was suspended in Mueller-Hinton broth medium and standardized to a final concentration of 1 × 108 CFU/mL (0.5 McFarland standard). The standardized inoculum was diluted 1:100 to achieve a final bacterial concentration of approximately 5 × 105 CFU/mL in each well of a 96-well plate. We tested serial dilutions of naringin (Nar) and hydrolyzed naringin (NH) at concentrations ranging from 391 to 50,000 µg/mL, while silver nanoparticle (AgNP) concentrations ranged from 0.625 to 40 µg/mL. After 20 hours of incubation at 37°C, we calculated the bacterial growth rate percentage on microplates by measuring optical density (OD) at 620 nm using a Multiskan FC spectrophotometer (Thermo Fisher Scientific). Absorbance values were adjusted to account for compound-specific absorption in the medium. The growth inhibition percentage was calculated using the formula: [(Control Negative OD – Sample OD) / Control Negative OD] × 100. MIC was defined as the lowest concentration preventing bacterial growth and expressed in µg/mL. Each test was performed in triplicate wells and repeated in three independent experiments.
For determining the minimum bactericidal concentration (MBC), we plated 20 µL aliquots from each non-turbid well following MIC assessment onto fresh media. These plates were incubated for 24 hours at 37°C. The MBC was defined as the lowest concentration at which no bacterial growth occurred on the media.
Additionally, the IC50 was calculated as the concentration that causes 50% inhibition, determined from the mean mortality across three replicates at each concentration, expressed as a percentage of the inoculum. A dose-response curve was generated by plotting the logarithm of the concentrations against the corresponding inhibitory effects. The equation of the resulting linear regression was then used to calculate the IC50 value.
The study included both positive and negative controls. Positive controls consisted of 2% chlorhexidine digluconate (CHX) and 5.5% sodium hypochlorite (NaOCl), which are commonly used irrigating solutions in clinical practice. The negative control consisted of bacterial broth, while fresh brain-heart infusion (BHI) broth served as a quality control for each experiment.
2.5. Checkerboard Assay
With the aim of evaluating the effect of naringin compounds and AgNPs combined, we used the checkerboard assay in a 96-well microplate following methods indicated by Bellio P et al. [25]. For this essay, MIC values were used to create combinations. The percentage of growth in each well was calculated as: (OD compounds combination well – OD background) / (OD compound free well – OD background) × 100. MIC for each combination was calculated and defined as the lowest drug concentration, which reduced bacterial growth by more than 80%. The combination index (CI) was calculated using cell death percentage as the effect and the strategy of Loewe additivity by the following equation: CI = EAB/EA + EAB/EB, where EA and EB are the independent observed effects, and EAB is the effect combined. [26]. CI=1, <1, and >1 indicate additive effect, synergism, and antagonism, respectively.
| Target Gene | Forward Primer | Reverse Primer | Product Size (bp) | Reference Sequence |
|---|---|---|---|---|
| Genes related to biofilm formation | ||||
| ace | TGGTCCAATTTCGTTTGGCG | AATTGAGGTCATCTCTCCTTGAT | 100 | NZ_KB944666.1:1229474-1230801 |
| agg | TCACTTGCCGAGTTTGAGCA | TGTTGGCCTGCTTCTGTGAT | 146 | NZ_KZ846041.1:719573-720814 |
| asa1 | GGCCAGTCAAGCCTTTTTGG | TAAACGTCTCCTGCCGCAAT | 108 | NZ_KB944668.1:3395-7285 |
| efaA | AGGCGGAAATGGCTGGTTTA | TGTTCTTGACCGGCACTTGT | 120 | efaA consensus |
| gelE | TAGCAGGTGAAGCAGGTGTG | GATAATCGCAGGCGCGTTTT | 131 | NZ_KB944666.1:c1884020-1882488 |
| gls24 | TAATTGTCGGCGGGGTCATT | TCCCATGTGCCTAAATGGCT | 130 | AJ000042.1 |
| sea1 | GTTCGCGAAAAGCTTGGGTT | GCTGCACGATTGATCGCATT | 128 | NZ_KB944668.1:1-2513 |
| Genes related to stress protection | ||||
| NADH Pox | ACTTGATGCGTGGTCGTCAA | TCGGCAGCTTCAATCCCAAT | 112 | NZ_KB944666.1:1337933-1339276 |
| ohr | GGACGTGCAGGAGAAGTTCA | TGCACCGTTAAAGCAAGCAC | 135 | NZ_KB944666.1:616100-616501 |
| sodA | GGCCACGCAAACCATACATT | GCCAGTTGCAGCTGTTTTGA | 144 | NZ_KB944666.1:623991-624599 |
| Reference gene | ||||
| recA | CGACTAATGTCTCAAGCACTAC | CGAACATCACGCCAACTT | 106 | NZ_KB944666.117 c93721-92675; Zheng et al. 2017 |
2.6. Time-antibacterial Activity
2.6.1. Ex Vivo Assay
To assess the ex vivo antibacterial efficacy of naringin, hydrolysed naringin, and AgNPs against E. faecalis, sixty freshly extracted single-rooted human teeth were selected based on periapical radiographs. Root canal preparation involved drilling and removal of dental pulp using round burs and a manual system with a Maillefer K-file 25 mm and Reciproc endodontic rotary files. The root canals were irrigated with 5,25% hypochlorite between files. The samples were then immersed in distilled water for 24 hours, rinsed with tap water, dried in a heating cabinet, and sterilized in an autoclave at 121°C for 20 minutes.
The teeth were randomly divided into subgroups and inoculated with an E. faecalis suspension at a concentration of 1.5x108 CFU/mL – 0.5 McFarland scale and incubated for 2 days. The presence of E. faecalis infection was confirmed by selecting one specimen for cell count density. A conventional irrigation protocol was followed using a 27G needle with 15 mL of each irrigating solution. Naringin solutions were prepared in sterilized distilled water and 5% dimethyl sulfoxide for dilution. The positive control groups consisted of NaOCl at 5.5% and chlorhexidine digluconate at 2%; the negative control was a sterile saline solution (sodium chloride at 0.9%).
After 72 hours, colony-forming unit (CFU) counting was performed to assess the efficacy of irrigation. Each root canal was rinsed with 200 µL of sterile saline solution, and 100 µL of the rinse was collected for serial dilution to estimate the number of E. faecalis colonies.
2.7. RNA Extraction and cDNA Preparation
Changes in E. faecalis virulence gene expression were assessed in bacterial cultures after exposure to each compound individually: naringin (50,000 µg/mL), hydrolysed naringin (50,000 µg/mL), and AgNP (33 µg/mL) in BHI broth compared to the control. After 24 hours of incubation, bacteria were collected by centrifugation (2,500 g for 5 minutes at 4°C). The universal RNA purification kit (EURx, Poland) with DNaseI treatment was utilized to isolate bacterial RNA following the manufacturer's instructions. RNA concentration was measured using on Qubit 2 fluorimeter (Thermo Fisher Scientific, USA). The target RNA fragments were then converted into cDNA using smART reverse transcriptase (EURx, Poland) and the set of custom reverse primers in a total reaction volume of 25 µL (Table 1).
2.8. Real-time Quantitative PCR
Promega GoTaq™ qPCR Master Mix (Thermo Fisher Scientific, USA) was used in subsequent qPCR reactions in a total volume of 30 µL. The cycling conditions were incubation at 50°C for 2 min, polymerase activation at 95°C for 10 min, followed by 40 cycles at 60°C for 30 s. The qPCRs were performed in triplicate. Each PCR set included a no-template control and a positive control. Most primers were redesigned to obtain unique PCR products of predicted size below 150 bp (Table 1). Relative expression levels of selected target genes were calculated using the delta-delta Ct (2−ΔΔCt) method with recA (recombinase protein A) as a reference calibrant gene [27, 28]
2.9. Transmission Electron Microscopy (TEM) Preparation and Analysis
Overnight, Mueller-Hinton agar cultures were subcultured in BHI broth to the exponential phase. The bacteria were then diluted into fresh medium to a final concentration of 1x108 CFU/mL and placed in Eppendorf tubes in 1 mL aliquots, each supplemented with a combination of naringin, hydrolysed naringin, and AgNPs. After 20 hours of incubation, the cells were centrifuged and immediately fixed with 2% glutaraldehyde. The samples were viewed using a JEOL-JSM 6490LV scanning electron microscopy (termoionic) instrument at the University of Antioquia.
2.10. Statistical Analysis
Statistical analyses were conducted using GraphPad Prism (v.10.0). To ensure the reproducibility of the results of the in vitro study, the compounds were evaluated using triplicates within each assay at three independent times. Data were shown as mean and Standard deviation (SD) values. Data were explored for normality using the Shapiro-Wilk test. The antimicrobial efficacy data for each compound were analysed by qualitative susceptibility test and quantitative MIC test using the microdilution method. MBC/MIC ratio was used to determine whether an antibacterial agent is bactericidal or bacteriostatic. A ratio ≤4 indicated bactericidal.
The ex vivo assay involved a total of sixty teeth, with 10 specimens in each group. We calculated the sample size based on the results from the in vitro assay. We used www.statulator.com for the sample size calculator, comparing two independent means and providing an expected difference in absorbance (OD620 nm) of 0.168 and an expected SD of 0.126. To evaluate the effect of irrigation on the compounds, the Mann-Whitney U test was used. Comparisons of the value of bacterial count (mean ± SD) were made between each group and the no-treatment group. A p-value of less than 0.05 was deemed statistically significant.
3. RESULTS
3.1. Characterization of Chemically Synthesized AgNPs
Characterization of AgNPs was conducted through concentration and morphological analysis. Studies on the concentration of silver in AgNP samples revealed that considering the original sample concentration of 40 ppm, approximately 70% of the sample consisted of nanoparticles, corresponding to 27.9 ppm per sample. This indicates that the majority of the sample is made up of AgNPs, and their concentration is significantly higher compared to other components present in the sample.
Transmission Electron Microscopy (TEM) analysis confirmed that 80% of AgNPs synthesized using citrate exhibited a spherical and quasi-spherical morphology, with dispersed particles also observed. The particles typically had a size distribution of 14 nm ± 0.4, as shown in the TEM images (Fig. 1A). Particle size was determined in situ using Dynamic Light Scattering (DLS), which measures nanoparticle size in solution. In this case, three peaks were observed in the DLS data (Fig. 1B), indicating different nanoparticle sizes: 2.03 nm, 15.15 nm, and 42.67 nm, respectively. The average nanoparticle size was found to be 19.95 nm. The synthesized nanoparticles were polydisperse with an index of 0.817, suggesting that they could have a broad size distribution [29]. However, the majority of nanoparticles were below 20 nm in size.
The X-ray diffraction (XRD) analysis confirmed that the silver nanoparticles possessed a crystalline structure (Fig. 1C). The diffraction peaks or angles at 2θ of 38.17°, 44.34°, 64.52°, and 77.44° corresponded to the lattice planes (111), (200), (220) and (311), respectively, suggesting a face-centred cubic (FCC) crystal structure [30]. These planes
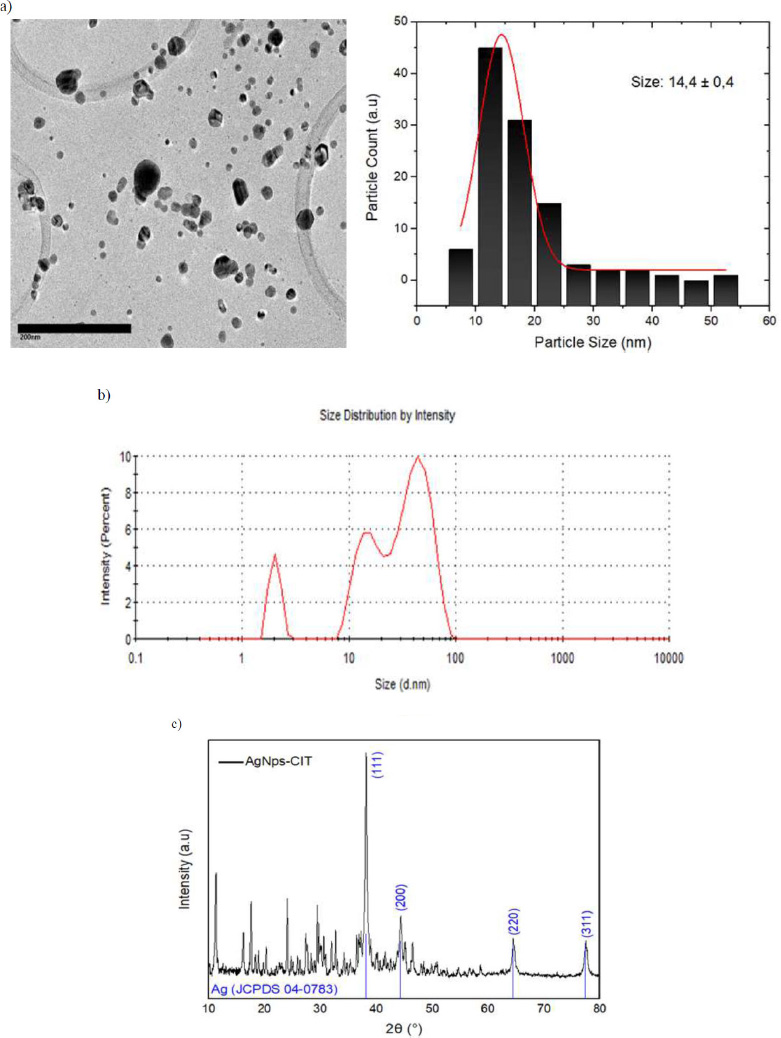
TEM images of AgNPs-CIT nanoparticles. a) Size distribution of AgNPs-CIT. b) DLS of AgNPs-CIT. c) XRD of AgNPs-CIT.
were compared with the crystallographic data from the JCPDS-04-0783 database. The particle sizes in the XRD patterns were calculated using the Scherrer equation, revealing that for AgNP-CIT, the particle size was determined to be 17 nm based on the maximum width of the diffraction peaks. This value is slightly higher than the one obtained in the TEM analysis (± 3 nm).
3.2. In Vitro Antibacterial Activity of Naringin, its Hydrolysed Derivatives (NH), and AgNPs against E. faecalis
Qualitative assessment of antimicrobial activity showed MIC at 12,500 μg/mL, 25,000 μg/mL, and 7 μg/mL for naringin, hydrolised naringin, and AgNPs, respectively. MBC was not detected for naringin compounds. MBC for AgNPs was 28 μg/mL. Data suggest a bacteriostatic behaviour for naringin and NH against E. faecalis. However, the MBC/MIC ratio could not be calculated for validation. IC50 was 21,500 μg/mL and 26,000 μg/mL for naringin and NH, respectively (Table 2).
The percentage of E. faecalis growth rate after 24 hours of incubation at different serial dilutions of naringin, NH (prunin + naringenin), and AgNPs was recorded in Figs. (2a, b, and c). Values above zero indicate no growth and reduction in final absorbance vs initial absorbance. Results evidenced a reduction of 50% in growth rate with concentrations greater than 12,500 μg/mL for both naringin and NH compounds. From the same data dose-response curve showed a quantitative MIC value of 25,000 μg/mL and 12,500 μg/mL for naringin and NH, respectively. For AgNPs, the growth rate inhibition was 100% for all concentrations. Then, according to data from Fig. (2c), we defined a MIC value of 7 μg/mL.
| - | Naringin (μg/mL) |
Hydrolysed Naringin (μg/mL) |
AgNPs (μg/mL) |
|---|---|---|---|
| MIC | 12,500 | 25,000 | 7 |
| MBC | N.I | N.I | 28 |
| IC 50 | 21,500 | 26,000 | N.D |
| MBC/MIC ratio | ND | ND | 4 |
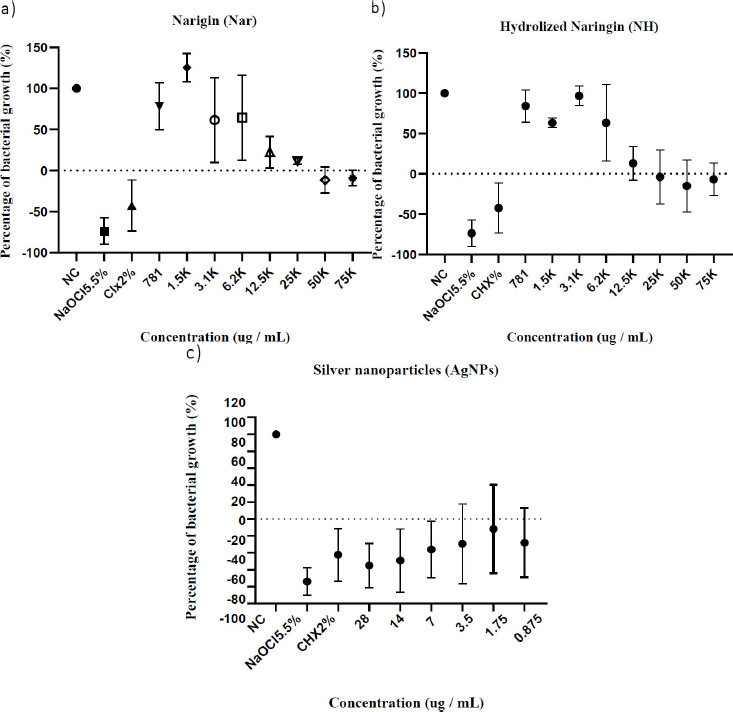
Enterococcus faecalis growth rate in presence of the compounds. All data shown are average growth percentage (% ± SD) from three biological replicates. Percentages below zero show percentage rate of reduction in absorbances from initial measurements.
| Naringin | Hydrolysed Naringin | ||
|---|---|---|---|
| CI ± SD | Combination | CI ± SD | Combination |
| 2.6 ± 0.5 | Nar50 AgNPs3.5 | 1.5 ± 0.3 | NH50 AgNPs14 |
| 2.5 ± 0.7 | Nar50 AgNPs7 | 1.7 ± 0.2 | NH25 AgNPs14 |
| 2.8 ± 1.3 | Nar25 AgNPs7 | 2.1 ±0.8 | NH12.5 AgNPs14 |
| 1.2 ± 0.3 | Nar12.5 AgNPs14 | 2.0 ± 1.0 | NH50 AgNPs7 |
| - | - | 2.3 ± 0.2 | NH50 AgNPs3.5 |
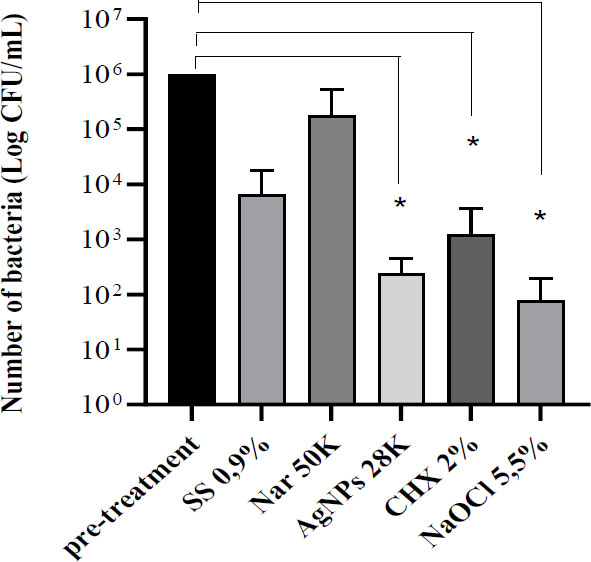
Effect of irrigation with the different compounds. Bars represent the average Log bacterial count (CFU/mL) ± SD from ten teeth on each group. SS 0,9%= irrigation with sodium chloride 0.9% as the negative control.
3.3. Compounds Combination
We defined matrix interaction considering the following concentrations of the compounds: X2 MIC (doble value of MIC), MIC, ½ MIC, and ¼ MIC for naringin compounds, and X2 MIC, MIC, and ½ MIC for AgNPs. X means a number of times. The resulting combinatorial MIC was naringin at 12,500 μg/mL and AgNPs at 14 μg/mL. Table 3 shows the results of ICs for combinations that reduced bacterial growth by more than 80%. All data shows CI values above 1, meaning an antagonistic effect.
3.4. Ex Vivo Antibacterial Activity of Naringin and AgNPs against E. faecalis in Root Canals
In extracted human premolar teeth, a concentration of 28 μg/mL of AgNPs was tested to examine ex vivo antibacterial efficacy. Naringin was tested at 50,000 μg/mL. Hydrolysed naringin was not evaluated because solutions with concentrations higher than 50,000 μg/mL were too dense to pass through the irrigation needle. After 72 hours, complete inhibition was not observed in teeth with more than 1x10ˆ5 CFU/mL in the root canal. Naringin did not show a significant reduction in the number of bacteria. AgNPs had results similar to those of common dental irrigants such as NaOCl and chlorhexidine (Fig. 3).
3.5. Expression of E. faecalis-RNA Virulence Factors
Ten virulence genes associated with pathogenesis were detected in Enterococcus species, including seven genes related to biofilm formation: ace (collagen adhesion protein to dentin), agg (aggregation substance), asa1 (adhesin gene), efaA (endocarditis-specific antigen A), gelE (ATP-dependent zinc metalloprotease FtsH), gls24 (protein cell morphology), and sea1 (surface exclusion protein Sea1/PrgA). Three genes were related to stress protection: NADH Pox (NADH peroxidase), Ohr (hydroperoxide resistance gene), and sodA (superoxide dismutase).
The changes in gene expression were analysed in bacterial cultures exposed to NH, naringin applied at a concentration of 50,000 μg/mL, and AgNPs at 28 μg/mL (Fig. 4). In the ∆∆Ct approach, agg and efaA genes responsible for biofilm formation showed relative expression below 1 and were downregulated in E. faecalis after all the treatments mentioned above. qPCR data showed that exposure to AgNPs for over 20 hours resulted in the downregulation of all tested genes responsible for biofilm formation and stress protection (Fig. 4). Supplementation of media with 50,000 μg/mL naringin resulted in a 100-fold increase in the asa1 gene and over a 10-fold increase in gls24 and NADH peroxidase. Increased expression induced by naringenin was also found in the sea1, ohr, and sodA genes. These results suggest that naringenin at a bacteriostatic concentration activates stress protection mechanisms and induces part of the biofilm formation genes (asa1, gls24, and sea1). Exposure to NH at 50,000 μg/mL resulted in a 9-fold increase in ace gene expression.
3.6. Nar+AgNPs Cause Alterations in the Morphology of E. faecalis
Fig. (5) represents, in the presence of AgNPs, we observed bacterial cells that exhibited altered morphology with misplaced septa and some bumps. Combining Nar+AgNPs also disrupted the typical pairs (diplococci) and their organizational structure, showing greater bacterial aggregation and a round shape. These results align with the observed down-regulation of gls24 gene expression upon exposure to AgNPs.
4. DISCUSSION
E. faecalis poses a significant threat to recurrent root canal treatment failure due to its increased antibiotic resistance and broad pathogenic mechanisms [1]. Studies have shown that flavonoids such as naringin have a wide range of beneficial properties for human health, including frequent reports of antimicrobial activity [31-33]. Flavonoids can provide an antibacterial effect by affecting transcription, impairing cell membranes, disrupting metabolic pathways and the generation of reactive oxygen species, and inhibiting toxin release, cell adhesion, or biofilm formation [34, 35]. Silver nanoparticles, obtained by chemical reduction or even through green synthesis [36], have also displayed antibacterial activity against many pathogenic bacteria [37]. Its activities have shown synergistic antibacterial activity and strong antioxidant potential [38]. Recently, researchers have evaluated the combination of metal nanoparticles with flavonoids in the form of flavonoid-silver nanoparticle conjugates. Different bioflavonoids, such as myricetin, hesperidin, quercetin, and others, coated with nanoparticles, have demonstrated potent antibacterial activity [21]. The combination of naringin with silver nanoparticles has not been evaluated. This study aimed to evaluate a better combination of natural antimicrobial agents like naringin with the potent antibacterial properties of AgNPs against E. faecalis, which go beyond their function as carriers for flavonoids or other molecules.
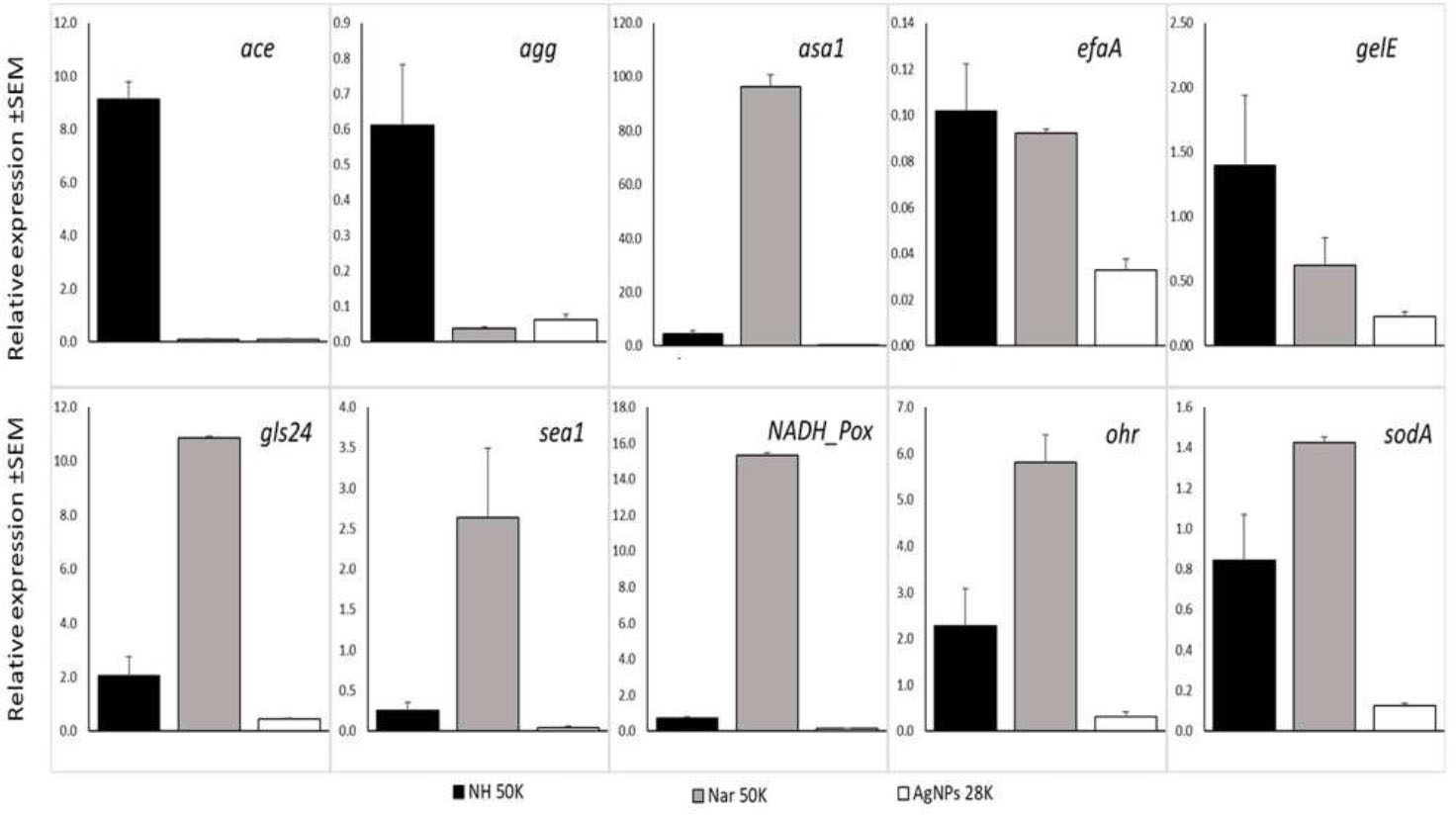
Expression of virulence-associated genes related to biofilm formation and stress protection from E. faecalis. Ace - adhesion to dentin, agg - aggregation substance, asa1 - mediates aggregation, efaA - endocarditis specific antigen, gelE - M4 family metallopeptidase coccolysin, gls24 - protein cell morphology, sea1 - surface exclusion protein SEA1/PrgA, NADH_Pox - NADH peroxidase, Ohr - hydroperoxide resistance, sodA - superoxide dismutase in respect to reference gene: recA - recombinase protein A.
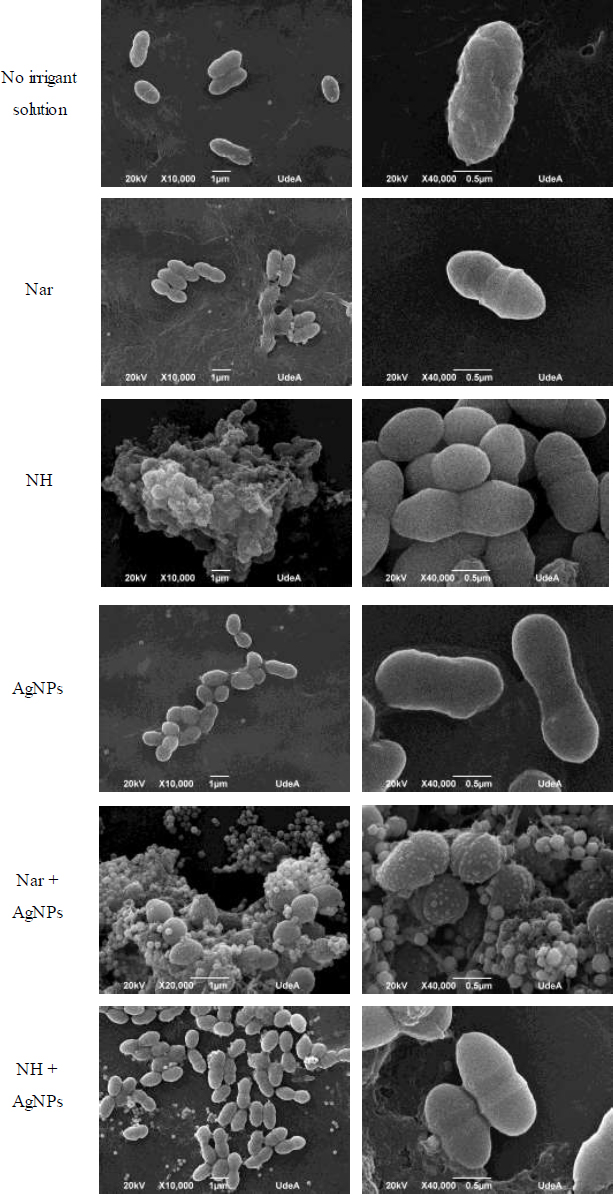
Scanning electron microscope images of E. faecalis after overnight exposure to compounds. White bars represent 10,000 and 40,000X. The images shown are representative of two experiments.
In our current study, we designed a novel combinatorial compound system to mix and potentiate compound properties. In vitro results showed that naringin and AgNPs, either alone or together, can effectively stop the growth of E. faecalis. However, naringin did not have any antibacterial properties. Naringin and its aglycone derivatives, prunin, and naringenin, required concentrations higher than 10,000 μg/mL to achieve inhibitory activity, as previously reported by other authors [39, 40]. For other Gram-positive bacteria, such as Staphylococcus aureus, MIC concentrations for inhibiting cell growth have been 1,000 μg/mL [11]. Concerning structural changes, it has been described that the main site of flavonoid action on Gram-positive bacteria is the phospholipid bilayer [9] and that the degree of antibacterial activity of bioflavonoids is related to the structure of the flavonoid [41]. Veiko et al. studied the effect of naringenin on Staphylococcus aureus and found that it did not alter cell diameter or disrupt the structural organization of cell membranes at concentrations ranging from 10 to 100 μM [42]. Consistent with those findings, we did not identify any specific morphological alterations in E. faecalis related to naringin or its derivatives.
Bacterial growth exhibited a slight, although not statistically significant, enhancement relative to the negative control at naringin concentrations below 3,100 μg/mL. We hypothesize that remnant sugars, rhamnose, and glucose, produced during the hydrolysis of naringin by naringinase, could benefit bacterial growth in the BHI medium culture [43]. For Enterococcus and other Gram-positive bacteria, rhamnose is an important constituent of their cell wall [44].
AgNPs are known to be antimicrobial agents, and this study demonstrated bactericidal activity consistent with previous studies [45, 46]. AgNPs at a concentration of 14 μg/mL exhibited efficacy against E. faecalis efficacy comparable to 2% chlorhexidine and 5.5% NaOCl, which were used as positive controls in this study. These results are in line with those reported by Duque-Aristizabal et al. [47], where AgNPs immobilised in zinc oxide-eugenol cement, an endodontic sealant known for bacterial inhibition, also showed bactericidal activity.
The combination of naringin or its aglycone derivatives with AgNPs did not enhance the antimicrobial effect against E. faecalis; in fact, naringin appeared to reduce the bactericidal potential of AgNPs. The exact mechanism by which AgNPs act against bacteria is not fully understood, but some hypotheses suggest that the generation of reactive oxygen species and the release of Ag ions denature proteins [48].
Naringin and other flavanones have been used in the biological synthesis of silver nanoparticles [49]. However, when in contact with AgNPs, they may lead to a reduction of Ag ions and the agglomeration of nanoparticles, as observed in the transmission electron micrographs obtained in this study, resulting in a decrease in the bactericidal activity of AgNPs. Additionally, a change in the intensity of the colour of the reaction medium was detected, indicating the need for further investigation to clarify and explain this finding.
In the current study, human teeth extracted for the ex vivo experiment simulated clinical conditions during irrigation procedures. Like in vitro experiments, AgNPs exhibited effective antibacterial efficacy compared to 2% chlorhexidine and 5.5% NaOCl. This result closely aligns with findings by Halkai et al. [45], who reported a MIC of 30 mg/ml for AgNPs against E. faecalis. In another study, Wu et al. [50] evaluated AgNPs as an irrigant against E. faecalis biofilm on root dentin; the results indicated that efficacy depends on the mode of application.
Naringin did not ensure the reduction of bacterial load or inhibition of bacterial growth after 72h under specific conditions of the root canal system, possibly due to its limited solubility at 50,000 μg/mL, which hinders debridement or adequate suction of the solution. Further studies are needed to optimise the preparation of the naringin irrigant solution, as well as other established parameters such as application time, volume, and activation methods [51].
Considering the expression of virulence genes, their presence has been associated with pathogenicity among the most important strains of Enterococcus species [52]. Many previous studies have reported modification in the expression of different virulence genes in E. faecalis after exposure to different stress conditions because those genes allow this microorganism to survive under harsh conditions [53, 54]. Unlike the use of flavonoids as an antimicrobial agent, in the case of E. faecalis as an important probiotic microorganism in the intestinal tract, there is evidence of the use of flavonoids as a main bioactive component in Cyclocarya paliurus to alleviate or improve tolerance to stress conditions in the gastrointestinal environment [55]. E. faecalis deals with reactive oxygen species and neutralizes the environment for survival by synthesizing superoxide dismutase and peroxidases or by using oxidative repair strategies [56]. We found that exposure of E. faecalis to naringin induced high expression of genes related to survival under stress conditions (15-fold NADH peroxidase and 6-fold Ohr).
Environmental factors also play a role in regulating the mechanisms of enterococcal gene expression, which are related to toxin production, surface adherence, and cell signalling [57]. The levels of virulence gene expression also depend on the tissue and growth phase. Different genes involved in virulence showed significant changes in expression during the log or stationary phase in serum and urine. The relative expression (RC) levels of ace (RC=9) and gls24 (RC=11) genes found in our study were within the range of respective genes found in serum and urine [57]. The gls24 gene is a protein involved in morphological changes that make bacteria more resistant to stress challenges; its disruption causes growth and morphological defects [58]. This is consistent with our observations of a conserved morphology of the coccal pairs in the naringin group compared to the slight morphological changes in the AgNPs group.
Sea1 and asa1 are independently transcribed genes that encode a surface exclusion protein and aggregation substance, respectively. We found that the application of naringenin induces the expression of the Asa1 gene. This gene promotes biofilm formation by aggregation between bacteria for the transfer of plasmids [28]. However, simultaneous maximal transcription of asa1 and the presence of aggregation substance on the cell was observed 15-20 after induction [59]. Therefore, high expression of Asa1 in naringenin-containing media at the stationary phase may result from a delay in biofilm formation.
Detailed relative expression analyses may require different reference genes, and recA was used as a reference to establish expression levels of gene expression analysis. The recA gene of E. faecalis codes a protein that is important for the survival of bacteria. This protein regulates response systems to DNA damage [60] and protects bacteria from harsh environments such as oxidative stress and antibiotic treatment [61]. As far as we know, it is the first report that bacterial inhibitory concentrations of bioflavonoids can also alter gene expression in ways that help biofilm formation.
This study had some limitations, including difficulties achieving complete solubility of naringin in the ex vivo assay. We used 2% DMSO to prepare the naringin irrigant solutions to minimize any interference or additional antimicrobial activity from the solvent. This, limited similar conditions at the time of the irrigation process for root canals, and small particles might have remained in the canal, allowing the persistence of bacterial cells into root canals. Also, in this study, checking how well individual and combined compounds fight biofilm was not planned at first, but it could support the findings from the results obtained from RNA expression.
CONCLUSION
We identified naringin and its derivatives' bacteriostatic potential at MIC concentrations exceeding 12,500 μg/mL. This bacteriostatic effect allows some bacterial cells to remain and proliferate after root canal irrigation processes. In vitro, combining effective antimicrobial concentrations with silver nanoparticles showed no synergistic effect; on the contrary, we observed antagonism. Results of naringin compounds' effects on bacterial virulence gene expression revealed enhanced protective mechanisms against oxidative stress and biofilm formation. These findings suggest that naringin should not be used in combination with AgNPs in endodontic irrigant solutions. The compounds require higher concentrations while providing no additional antimicrobial activity. Although individual use of naringin exhibits bacteriostatic properties, it could potentially enhance bacterial resistance to root canal conditions. The impact on biofilm formation requires further investigation.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: J.S.V.: Study concept and design; M.A.M., D.L., M.T., J.M.G.: Methodology; J.M.M., D.A.M.: Data collection; J.P.V; Experimentation. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ICP | = Inductively Coupled Plasma |
| GFAAS | = Graphite Furnace Atomic Absorption Spectroscopy |
| DLS | = Dynamic Light Scattering |
| XRD | = X-ray diffraction |
| OD | = Optical Density |
| CHX | = Chlorhexidine Digluconate |
| BHI | = Brain-heart Infusion |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the institutional ethic committee of Universidad Antonio Nariño No.2021025 (July 27th, 2021).
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
This work was supported by an Internal call funding Grant by Vicerrectoria de Ciencia, Tecnología e Inovación of the Universidad Antonio Nariño, Project Code: 2021025; and the external cooperative funding from the Department of Biotechnology and Bioinformatics of Rzeszów University of Technology, through Erasmus+ Staff mobility agreement.
ACKNOWLEDGEMENTS
Declared none.


