All published articles of this journal are available on ScienceDirect.
Inhibitory Effect of Shikimic Acid on Biofilm Formation and the Expression of Critical Biofilm-associated Genes in Streptococcus agalactiae
Abstract
Background
Streptococcus agalactiae is well-known for causing adverse pregnancy outcomes, such as stillbirths and miscarriages, as well as being a major cause of newborn sepsis and meningitis.
Aims
This study sought to investigate how shikimic acid influences the expression of genes involved in the synthesis of biofilms.
Methods
A total of 181 specimens were obtained from pregnant women. Twenty-two isolates were bacteriologically recognized as S. agalactiae from these specimens. They were gathered from hospitals in the Al-Anbar province. These twenty-two isolates were identified as Group B streptococcus (GBS) based on their cultural and microscopical properties, as well as automated (VITEK-2 system) and molecular identification based on the atr gene, which is an essential gene expressed in all S. agalactiae isolates.
Results
GBS produced varying amounts of biofilm (weak, moderate, and strong). Shikimic acid (SA) was tested for its antibacterial effect against biofilm. Shikimic acid was incubated at various doses, and its effects on biofilm growth and formation were evaluated by MTT and crystal violet assay. SA greatly reduced the GBS inhibitory effects on GBS biofilms in pregnant women. Furthermore, it demonstrated a potent initial cell attachment, but it had less inhibitory effects on biofilms that had already been developed on polystyrene surfaces after eight hours. However, the Checkerboard approach produced a synergistic interaction between erythromycin and shikimic acid. Polymerase Chain Reaction (PCR) was used to verify the presence of pilA and PilB genes in the GBS strain. We detected these genes by PCR. The results revealed that pilA was found only in three isolates (13.63%), but pilB was found in all isolates (22/22;100%).
Conclusion
Quantitative real-time PCR was used to determine the effect of shikimic acid on the expression of genes (sag1407 and sag1408), and the results showed down-regulation of the expression of biofilm synthesis genes sag1407 and sag1408 when treated with Sub-MIC of shikimic acid.
1. INTRODUCTION
Streptococcus agalactiae is an oval-shaped, catalase-negative, facultative anaerobe, Gram-positive cocci [1]. This group consists of 10 distinct serotypes, nine of which have been identified throughout history (Ia, Ib, II, III, IV, V, VI, VII, and VIII) and one of which was recently discovered (IX) [1]. Different serotypes are distinguished by type-specific capsular polysaccharides, which act as a virulence factor by which GBS avoids detection by the host immune system [2]. S. agalactiae is the most common cause of human newborn infections. It is a key contributor to illness in older adults and pregnant women who have underlying conditions like diabetes or immunosuppression. This organism, which is a natural component of the flora of the vaginal tract and gut, colonizes in 10-40% of pregnant women. GBS can result in urosepsis, chorioamnionitis, endometritis, pneumonia, and skin and soft tissue infections in both adults and pregnant women. Newborns with GBS develop meningitis, diarrhea, and sepsis [3]. GBS colonization and persistence in different hosts are dependent on their capacity to adhere to host cells and tissues. As a result, bacterial cell aggregation and biofilm formation are aided [4]. GBS strains differ in their ability to form biofilms, and these differences are linked to phylogenetic lineage, isolation source, and capsular serotype [5]. Biofilms provide defenses against hostile conditions, such as antimicrobials, high pH, and immune cells (D’Urzo et al., 2014).
The global expansion of antimicrobial resistance (AMR) poses a grave threat to global public health. AMR increases mortality and morbidity and places a substantial cost burden on health care. Due to the excessive use of antibiotics, antimicrobial medications eventually lose their efficacy [6]. S. agalactiae can acquire antibiotic resistance through a variety of mechanisms in addition to its inherent resistance to some medicines [7]. Shikimic acid (SA) is a (cyclohex-1-ene-1-carboxylic acid, (3R, 4S, 5R) 3,4,5-trihydroxycyclohexene-1-carboxylic acid, C7H10O5) that is soluble in water [8]. SA is isolated from the fruit of Japanese star anise (Illicium religiosum Sieb. et Zucc./Illicium anisatum L.) and derives its name from the Japanese word for this Asian plant, “shikimi”. Japanese star anise (Illicium japonicum) is harmful, whereas Chinese star anise (Illicium verum Hook.) is not harmful. Johann Frederik Eykman discovered SA as a chemical compound in 1885. To date, SA has been widely utilized in the pharmaceutical sector for the manufacturing of medications [9]. The major source of SA is the fruit of the evergreen Badian tree (Star anise) (Illicium verum Hook.). Due to the inefficiency of SA extraction from anise (3–7%), raw material from American ambergris seeds was also extracted. Additionally, SA can be produced by modifying the metabolism of Escherichia coli O6 [10]. Shikimic acid is a key component in producing the antiviral medication oseltamivir phosphate, marketed as Tamiflu® and used orally to treat influenza viruses [11]. SA has received unprecedented attention as an essential component in biological studies since it has anti-inflammatory, analgesic, antioxidant, antithrombotic, and antibacterial effects [12]. SA affects majorly gram-positive bacteria. The effect of SA on bacteria derives from the interaction of acid with lipids and proteins in the bacterial membrane [13]. This study aimed to study the effect of shikimic acid on gene expression of biofilm synthesis.
2. METHODS
2.1. Sample Collection
From the beginning of August, 2021 to the end of December, 2021, 181 specimens were taken from pregnant women who were in their third trimester. The specimens included vaginal swabs taken from female patients who were hospitalized in hospitals located within the Al-Anbar province. The methods used in this research were mentioned previously [14].
2.2. S. agalactiae Identification
Isolates were obtained from the vaginal swabs, and then they were cultured by spreading over agar containing 5% sheep blood. The plates were incubated at 37 °C and 5% CO2 for 18–24 hours. Gram stain and catalase test were used in conjunction with other standard micro- biological and morphological identification techniques for S. agalactiae in order to determine that the isolates were GBS. Moreover, CAMP, bacitracin tests, automated identification using the VITEK-2 system, and molecular identification based on the atr gene were also employed.
2.3. Evaluation of the Minimum Concentration of Inhibitor (MIC)
The MIC of shikimic acid was determined using the Resazurin Microtiter-plate Assay (REMA).
2.4. Synergism between Shikimic acid and Erythromycin
Erythromycin was combined with shikimic acid by utilizing the checkerboard technique. In 96-well microplates, the checkerboard method was employed to explore any potential synergistic interactions between erythromycin and shikimic acid.
2.5. Biofilm Development
Quantitative tests were utilized to measure the growth of biofilm, as described by Bertelloni, utilizing a microplate reader and 96-well sterile polystyrene microtiters with flat bottoms [15]. The effects of shikimic acid against biofilm formation were investigated.
2.6. Biofilm Biomass Assay
Cell attachment for S.agalactiae isolates was assessed using the modified crystal violet (CV) assay, as described by Djordjevic et al. (2002). A microplate reader was used to detect absorbance at 595 nanometers. Based on the following equation, the mean absorbance (OD595 nm) was used to determine the biomass formation inhibition percentage for each concentration of the test materials:
Percentage inhibition = 100 - [(OD595 nm experimental well with test material / OD 595 nm control well without test material)x 100] [16].
2.7. Biofilm Metabolic Activity Assay
The metabolic activity of the biofilm growth by S.agalactiae was examined using an MTT assay according to Schillaci et al. (2008). The absorbance was then measured at 570 nm using the microplate reader [17].
2.8. Determination of Biofilm Inhibitory Activity of Shikimic Acid
2.8.1. A-Inhibition of Initial Cell Attachment
The effects of inhibitors on early cell attachment during biofilm formation were also evaluated. Using two distinct microtiter plates, solutions of shikimic acid (corresponding to 0.25 MIC, 0.5 MIC, 1 MIC, and 2 MIC) were created. The MTT assay was utilized to evaluate the metabolic activity, whilst the modified crystal violet (CV) assay was employed to detect biofilm formation [18].
2.8.2. B-Inhibition of Preformed Biofilm
The effect of shikimic acid on biofilm development and maturity was estimated according to Sandasi et al. (2010). Before adding shikimic acid, biofilms were given 24 hours to form. The plates were incubated for 8, 12, 16, 20, and 24 hours following the application of shikimic acid to the biofilms. The biomass attachment of biofilms was then examined using a modified CV test, and MTT studies were conducted on biofilm cells that had already formed [18].
2.9. Isolation and Quantification of DNA
DNA isolation kits (Geneaid, Korea) were used to isolate genomic DNA from bacterial cultures according to the manufacturer's instructions. The concentration and purity of DNA were evaluated using a Nano-drop device, and then DNA was stored at -20 °C to prevent deterioration.
2.10. Conditions and Mixtures of PCR Reactions
Amplification of ATR gene was done using standard PCR and ATR primers, such as 5’-CAA CGA TTC TCT CAG CTT TGT TAA-3’ and 5’-TAA GAA ATC TCT TGT GCG GAT TTC-3’(Mudzana et al., 2021), gyrA primers, 5- CGGGACACGTACAGGCTACT-3,5-CGATACGAGAAGCT CCCACA-3, sag1407 primers 5- TGGTGACTTATGGACG-3, 5- TGTACCAATACCACCTG-3, and sag1408 primers 5-TTCGGCACAATAGGAGTTG-3 and 5- CTTAACTTGCCAA GTCTGG-3 [19]. Afterward, the 20 μl reaction mixture was prepared according to the manufacturer’s instructions (BIONEER, Korea). An initial denaturation step at 94 °C for 4 minutes was followed by 35 cycles of denaturation at 94 °C for 1 minute, annealing at 58, 52, and 53°C for 45 seconds, extension at 72 °C for 1 minute, and a final elongation step at 72 °C for 7 minutes.
2.11. RNA Extraction
According to the guidelines for the TRIzol™ Reagent, total RNA was extracted from treated and untreated isolates.
2.12. cDNA Synthesis
According to the oneScript® Plus cDNA synthesis kit, this synthesis was carried out under thermal cycler steps conditions: 25ºC 5 min, 60ºC 25 min, 85 ºC 5 min, 4ºC ∞.
2.13. qPCR Reactions Mixtures and Conditions
The total volume of the qPCR reaction mixture was 20 μL, prepared according to the real-time PCR program, which included initial denaturation at 95°C for 60 seconds (1 cycle), denaturation at 95°C for 15 seconds and extension at 60°C for 30 seconds (40-45 cycles), and a melt curve from 60°C to 95°C with varying time (1 cycle).
3. RESULTS AND DISCUSSION
3.1. Isolation of S. agalactiae
This study lasted four months, commencing in early August and concluding in late December, 2021. Twenty-two isolates were identified as GBS from 181 clinical specimens. The isolation rate of GBS from pregnant women was 12.15%, and the majority of the participants were between the ages of 25 and 37. In terms of clinical history, the individuals had multigravida (54.54%), abortion (22.73%), stillbirth (9.09%), or neonatal death (13.64%). Many factors influence the rate of S.agalactiae isolation from pregnant women, including isolate virulence and patient health state, environmental variables and hormonal changes that occur during pregnancy, and the consequent microbiota imbalance that increases the risk of GBS infections, which can result in difficulties for both mothers and children. Numerous local investigations demonstrated the prevalence of GBS in Iraq, such as Hassan et al., which reported an 18% prevalence of GBS in Baghdad. In addition, there are international studies that demonstrated GBS incidence rates and patient clinical histories. In Southeast Ethiopia, 75.8% of patients were multiparous, 25.3% had a history of abortion, 12.1% had a history of stillbirth, and 15.4% had a history of neonatal death, according to their clinical histories (Tesfaye et al., 2022). The prevalence rate was 17.89% in Egypt and 16.4% in Kuwait (Abdallah et al., 2021).
3.2. Identification of S. agalactiae
The findings of tests employing microscopic diagnostics to identify S. agalactiae were positive for Coccus (chain or pair) and negative for catalase. Blood agar is hemolytic for S. agalactiae. S.agalactiae is distinguished from other streptococcal species based on a major virulence factor utilized by GBS during pathogenesis, which is positive for bacitracin and the CAMP test. cfb gene encoded by the CAMP factor was positive in this instance, indicating that the colony tested was S.agalactiae. Given how widespread the cfb gene is in identifying GBS from other Streptococcus species, the CAMP test or PCR testing for the cfb gene is frequently used in GBS strains. Molecular identification of S. agalactiae was carried out by detecting the atr gene in all bacterial isolates (100%). The atr gene detection test is specific for GBS screening in pregnant women. It has a high degree of selectivity for S. agalactiae and encodes for an amino acid glutamines transporter that is unique to S. agalactiae. Due to the fact that it is a housekeeping gene, the likelihood of mutation is low (Schörner et al., 2014).
3.3. Evaluation of the Minimum Concentration of Inhibitor Using REMA Method
The MIC of shikimic acid was found to be 1.25 mg/ml.
3.4. Determination of the Effect of Synergism between Shikimic Acid and Erythromycin Using Checkerboard Technique
The emergence of drug-resistant bacteria has diminished the efficacy of conventional antibiotics, urging the development of alternative treatment methods for diseases caused by drug-resistant bacteria (Chi & Holo, 2018). In order to increase or restore antimicrobial action against multidrug-resistant bacteria, it is possible to create replacement antibiotics and discover or manufacture adjuvants (Montero et al., 2018). It is extraordinarily challenging to develop new antibiotics at the rate at which microbes develop antibiotic resistance strategies. The checkerboard approach was one strategy used to overcome the resistance of bacteria and make them responsive to currently available antibiotics (Ayaz et al., 2019). Synergy testing methods use susceptibility techniques to measure the cumulative efficacy of two or more compounds. Combinations of natural substances may enhance or facilitate the interaction of an antimicrobial agent with the pathogen, which would stop the development of resistance. Such inhibitors are useful for antibiotics linked to high resistance rates since lower concentrations of both drugs can be utilized in this method [20]. The objective of synergism is to reduce microbial resistance and toxicity, and the combination of antibiotics and natural products decreases the minimum inhibitory concentration (MIC) of antibiotics while boosting the sensitivity of multidrug-resistant bacteria to these medications (Newman & Cragg, 2016). Checkerboard assays of GBS demonstrated synergistic characteristics when erythromycin and shikimic acid were combined. The FICI values of 0.00972 indicate a synergistic effect between the tested substances. Through the synergistic interaction of natural compounds with currently available medicines, the phenomenon of antibiotic resistance can be efficiently dealt. The term “synergy” is applied when the combined therapeutic effect of two substances is larger than the sum of their individual effects. This study indicated that the combination of erythromycin and other substances produces synergistic effects, as measured by a decrease in metabolic activity, and restores sensitivity to erythromycin in erythromycin-resistant strains of GBS. Combining herbal medicines and phytochemicals with antibiotics and other therapeutically relevant pharma- ceuticals is, therefore, a relatively novel and effective technique for controlling resistant microbes. Several compounds have been investigated for their ability to alter microbial resistance, and several have been found to be effective against multiple targets, such as blocking PBP, enhancing bacterial outer membrane permeability, and inhibiting bacterial efflux pumps (Alani & AlMeani, 2022).
3.5. Biofilm Development
In the MTP assay, the characterization of S.agalactiae isolates ranged from strong (7/22; 31.81%), moderate (10; 45.45%), and weak (3;13.63%) to no biofilm producers (2/22; 9.09%). In contrast to our findings, another study conducted in 2016 found that only 13.8% of isolates in China produced biofilm (Jiang et al., 2016). Bacterial biofilms are an essential virulence factor that plays a crucial part in the pathogenesis of bacteria; this is owing to the greater resistance to host defenses that promote germ survival and proliferation (Rosini & Margarit, 2015). According to a study, S. agalactiae colonizing pregnant females is more capable of forming biofilm than GBS isolated from various symptomatic illnesses (Hany et al., 2018).
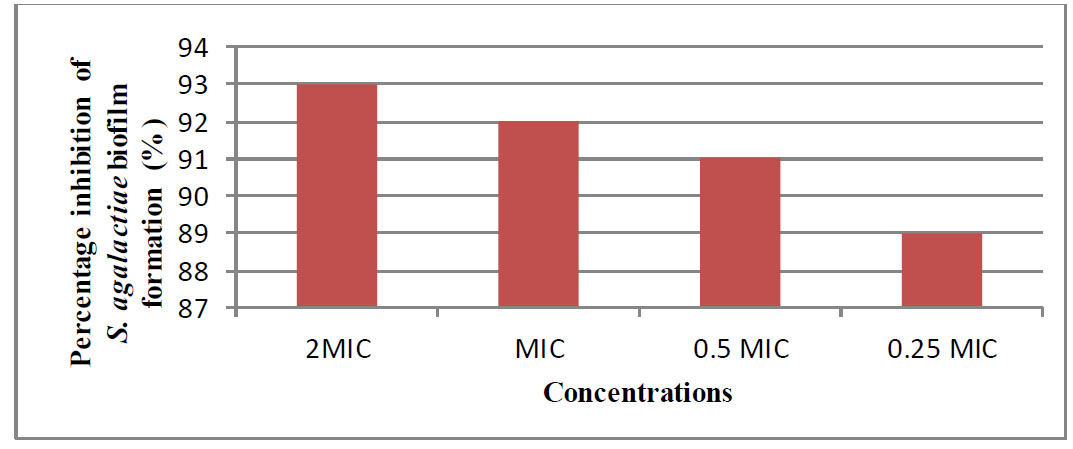
Result of different concentrations of shikimic acid on initial cell attachment of S.agalactia.
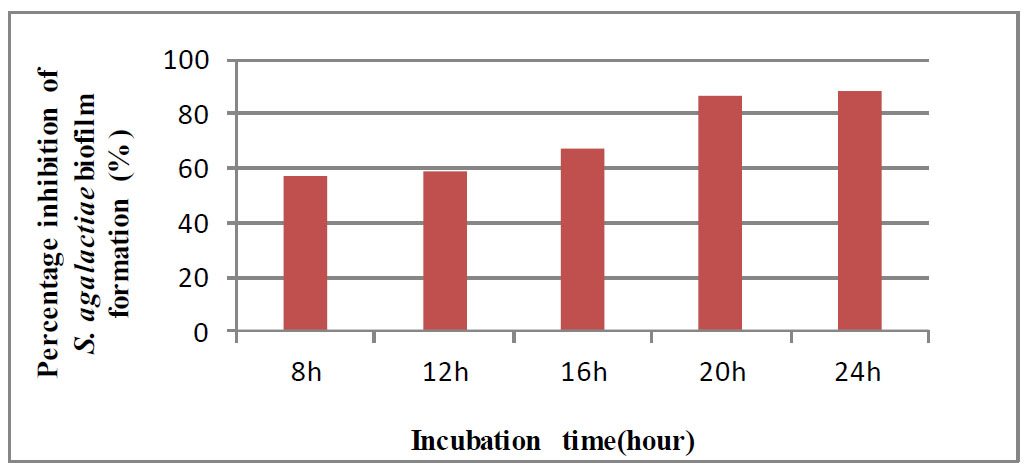
Percentage inhibition of GBS biofilm formation on preformed GBS biofilms.

Impact of shikimic acid concentration on GBS initial cell attachment metabolism.
3.6. Antimicrobial Activity against Sessile Cells
3.6.1. Determination of the Anti-biofilm Activity of Shikimic Acid on Biomass in S. agalactiae Biofilms
The anti-biofilm activity of shikimic acid on both the initial cell attachment and performed (24h) biofilms was examined. In a modified CV experiment, shikimic acid demonstrated a 93% greater impact on biomass attachment than control (percentage inhibition) at 2 MIC. At both MIC and 0.5 MIC, the inhibition was above 80%. While initial cell attachment was reduced, it was not as significantly inhibited as at 2 MIC or MIC, as shown in Fig. (1).
Despite employing 2 MIC of antibiofilms, it did not completely suppress cell attachment. Overall, the modification of biofilm formation sites with shikimic acid rendered them unsuitable for attachment, indicating that they are useful for preventing microbial adherence (Jadhav et al., 2013). Shikimic acid was tested against a biofilm that had already grown. An early phase of reversible (weak) attachment is followed by an irreversible (strong) attachment phase during the creation of biofilms [21]. The results demonstrated that the MIC of shikimic acid was evaluated against S. agalactiae preformed biofilm for 24 hours and determined by the CV assay. We noticed that the percentage inhibition of S. agalactiae preformed biofilm increased with longer incubation times, as shown in Fig. (2).
This reported resistance of a constructed biofilm can be attributed to the extracellular polysaccharide layer of the biofilm, which may halt the entry of antimicrobials, or to the mature biofilm's compact three-dimensional organization, which may prevent the entry of shikimic acid into the biofilm. As most antimicrobial substances are more effective against cells that are actively growing, this increasing resistance may also be influenced by this fact. Due to the poor growth rate of the cells in a biofilm and the deficiency of nutrients and oxygen, chemicals used to fight bacteria may have less impact [22].
3.6.2. Evaluating Antibiofilm Impact of Shikimic Acid against the Metabolic Activity of S. agalactiae Biofilms
MTT (thiazolyl blue tetrazolium bromide) was employed to detect connected living cells, while CV stains were found in both viable and nonviable cells. MTT, which is detectable calorimetrically, can only be converted into a colored chemical by living cells. The MTT assay detects only living cells based on their metabolic activity [23]. The MTT assay results revealed that shikimic acid considerably decreased the metabolic activity of S.agalactiae biofilms. Anti-adhesion action was the highest at 2*MIC, and inhibition began to diminish as the concentration of each antibiofilm lowered. Due to the low concentration of an inhibitor (0.25 MIC), biofilm formation must be inhibited with a high inhibitory concentration in order to prevent the formation, as illustrated in Fig. (3).
However, in the case of formed biofilms, shikimic acid reduced the metabolic activity of S. agalactiae at MIC. It has been demonstrated that the metabolic activity decreased with exposure time, culminating at 24 hours, as shown in Fig. (4).
3.6.3. Detection of Pili Biosynthesis Genes pilA (sag1407) and pilB (sag1408)
To verify the presence of pilA and pilB genes in the GBS strain, we detected these genes by PCR. The results revealed that pilA was found only in three isolates (13.63%), but pilB was found in all isolates tested (22/22; 100%).
Pili are cell-wall-anchored appendages that protrude from the surface of bacteria. Pili in GBS have been found to play a primary role in epithelial cell colonization, biofilm formation, translocation, and penetration of host cells. GBS pilus types are encoded by distinct genomic loci containing characteristics typical of Gram-positive pilus islands [24]. The GBS PilA type protein helps in the initial adhesion to the host. While the PilB type has been related to bacterial invasion and paracellular translocation, it has also mediated resistance to phagocytic killing [25].
3.6.4. RNA Concentration and Purity
RNA was extracted from S.agalactiae isolates before and after the treatment. Total RNA was extracted using TRIzol™ Reagent, and its concentration was from 22.5 to 85.6ng /μl.
3.6.5. Effects of Shikimic Acid on Gene Expression of sag1407 and sag1408 gene of S. agalactiae
To further confirm the inhibitory effects of the shikimic acid on S. agalactiae, strong biofilm-producing and biofilm-associated genes (pilA and pilB) were determined by quantitative RT-PCR. From the software of quantitative RT-PCR, Ct values of gene amplification were recorded. High Ct values mean low gene expression, while low Ct values show high gene expression. The calculation of gene expression fold change was carried out from the ΔΔCt value. The results of gene expression are shown in Tables 1 and 2 and Fig. (5).
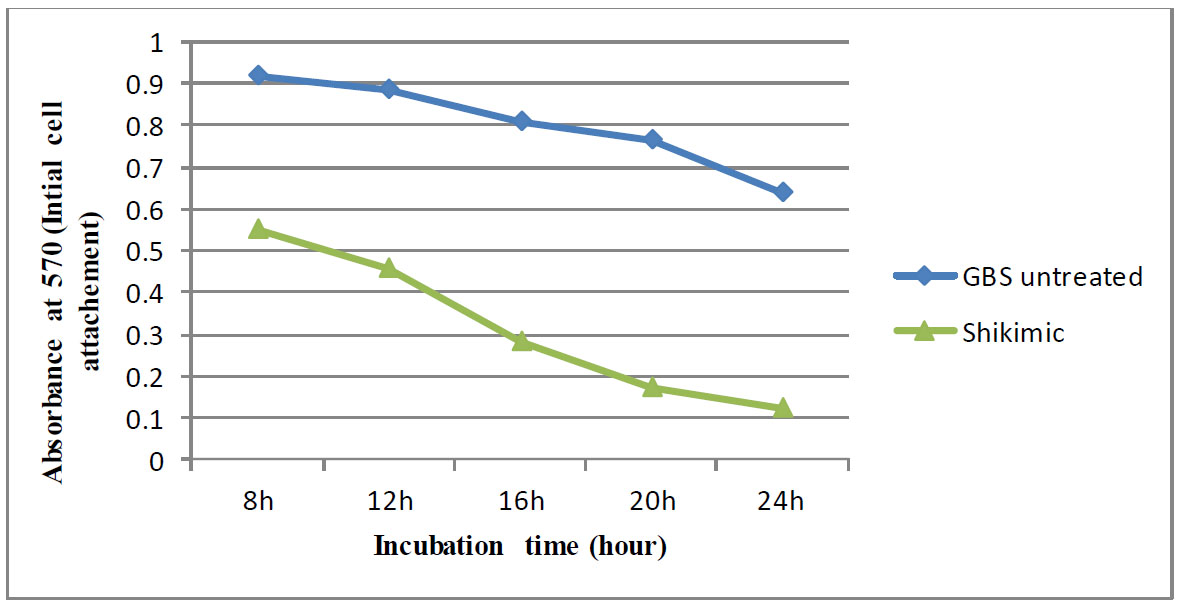
The impact of shikimic acid on the metabolic activity of S. agalactiae constructed biofilm cells was assessed using the MTT test after the cells were cultured for 8, 12, 16, 20, and 24 hours.
Table 1.
| Samples | Ct | ΔCt | ΔΔCt | Folding |
|---|---|---|---|---|
| Test treated with shikimic acid | 37.57 | 24.68 | 4.64 | 0.040107 |
| Reference treated with shikimic acid | 12.89 | |||
| Test untreated with shikimic acid | 33.59 | 20.04 | 0 | 1 |
| Reference untreated with shikimic acid | 13.55 |
| Samples | Ct | ΔCt | ΔΔCt | Folding |
|---|---|---|---|---|
| Test treated with shikimic acid | 14.07 | 1.18 | 0.75 | 0.594604 |
| Reference treated with shikimic acid | 12.89 | |||
| Test untreated with shikimic acid | 13.98 | 0.43 | 0 | 1 |
| Reference untreated with shikimic acid | 13.55 |
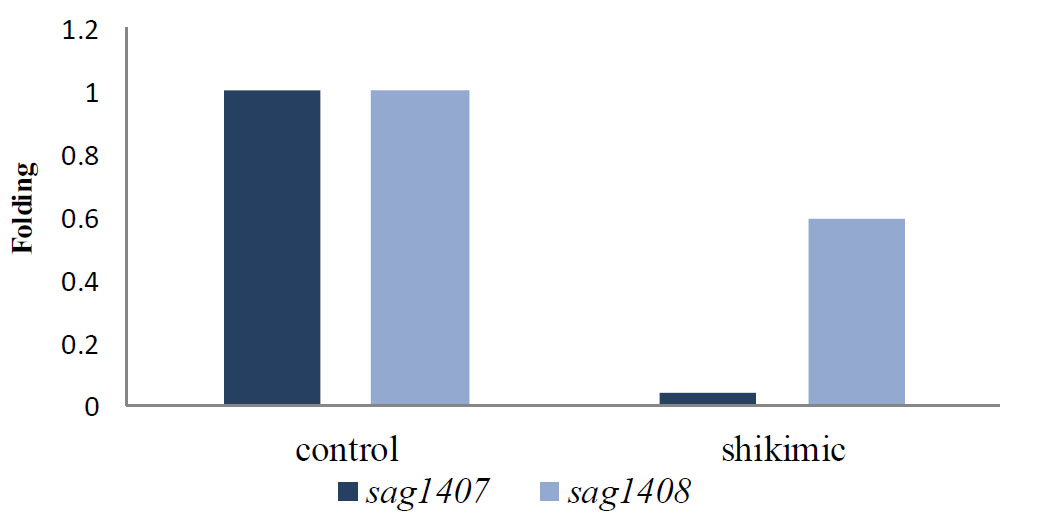
Gene expression of sag1407 and sag1408 genes before and after the treatment of S.agalactiae with shikimic acid.
Results demonstrated that after the treatment with shikimic acid, biofilm-producing S. agalactiae isolates had lower transcript levels of the pili biosynthesis genes (pilA and pilB) than the control isolates.
Shikimic acid affected the gene expression of the biofilm formation genes when treated with it, as it changed the CT value of each gene, and thus, this difference in value resulted in a variation in gene expression compared to the untreated sample. In this study, the CT value of the sag1407 gene was 33.59 before being treated with sub-MIC shikimic acid, and after treatment, the CT value increased to 37.57. Moreover, the Ct value for sag 1408 was 13.98 before the treatment and 14.07 after the treatment. However, for the reference gene, gyrA, the CT was 13.55 before treatment, and after treatment, it decreased and became 12.89. Furthermore, down-regulation was reported in the sag1407 gene expression levels when treated with sub-MIC shikimic acid, which reached 0.04011 compared to the control. Also, sag1408 reached 0.5946 than control. There is no available data regarding the investigation of the effect of shikimic acid on S. agalactiae. It was found that the treatment with shikimic acid decreased the expression of the pili biosynthesis genes (pilA and pilB), which are responsible for the formation of biofilms. This chemical compound exerted its antibacterial effect by disrupting cellular metabolism and destroying cell membranes. Meanwhile, this compound could inhibit biofilm formation and decrease toxin secretion and infection risk [26]. SA can inhibit EPS synthesis and modify the transcription of biofilm formation-related genes, resulting in fewer bacterial cells in its biofilm. The cell membrane and membrane proteins were also affected by SA interactions, harming the bacterial cells (Zhang et al., 2022).
SA demonstrated the capacity to obstruct bacterial cell movement. In microbial physiology, motility is significant because it is essential for adherence to the surface and subsequent biofilm development [27, 28].
In a study, it was reported that SA affects Staphylococci by down-regulating sarA, which is necessary for the formation of biofilms. Development of biofilms was reportedly suppressed in sarA mutants of S. aureus. Fibrinogen binding protein and other extracellular matrix proteins were reduced, and protease and nuclease activities increased in the mutants, which inhibited early cell-surface interactions and cell-cell interactions [29]. This study showed that SA mostly suppressed sarA transcription, preventing early biofilm development and establishment [30].
CONCLUSION
The frequency of GBS among pregnant women in Anbar, Iraq, and the colonization rate (12.15%) of this bacteria were studied recently. Shikimic acid contains antibacterial properties that are effective against S. agalactiae, an antibiotic-resistant biofilm. The combination of shikimic acid with antibiotics using the checkerboard technique against S. agalactiae could improve the susceptibility of this bacteria toward these antibiotics to treat infections resulting from multidrug-resistant bacteria. Shikimic acid, as a novel pharmaceutical compound, decreased the gene expressions of sag1407 and sag1408 genes for biofilm synthesis.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript’s content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| CV | = Crystal Violet |
| REMA | = Resazurin Microtiter-plate Assay |
| MIC | = Minimum Concentration of Inhibitor |
| SA | = Shikimic acid |
| AMR | = Antimicrobial resistance |
| PCR | = Polymerase chain reaction |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The current study originates from a medical student's thesis and has received approval from the Ethical Approval Committee of the University of Anbar in 2021 (No. 234).
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.


