All published articles of this journal are available on ScienceDirect.
Rapid In Vitro Regeneration and Genetic Fidelity Assessment of Regenerated Plants in Ayapana Triplinervis (Vahl) R.M. King & H. Robinson: An Ethnomedicinal and Ornamental Herb
Abstract
Background:
Ayapana triplinervis is a popular ethnomedicinal and ornamental plant species. Due to its high medicinal importance, A. triplinervis was recently documented in the French Pharmacopeia.
Objective:
Rapid and efficient tissue culture protocol development is crucial for the high production and biotechnological applications of this plant.
Methods:
In this study, an efficient tissue culture protocol was developed for plant regeneration using nodal explants of A. triplinervis. The nodal explants were treated in Murashige and Skoog’s (MS) medium supplemented with various individual concentrations of cytokinins (BAP and KIN) and auxins (IAA and IBA). The nodal explant was regenerated in three different steps: (1) initial shoot induction, (2) shoot multiplication and elongation, and (3) rooting.
Results:
The results revealed that all individual concentrations (10, 20, 30, or 40 mg/L) of BAP or KIN responded to induce shoot initiation. The highest shoot multiplication and elongation were achieved in the MS medium with 20 mg/L BAP and 20 mg/L KIN. The regenerated plantlets produced better roots on MS medium containing 1.0 mg/L of each IAA or IBA. The well-established rooted plantlets were maintained in the culture room and greenhouse for better acclimatization and achieved a 100% survival rate. We analyzed the genetic fidelity of in vitro regenerated plants using random amplified polymorphic DNA (RAPD) markers. No genetic polymorphisms were observed in vitro plants compared to the mother plants.
Conclusion:
This efficient protocol could benefit future biotechnological applications like mass multiplication, genetic transformation and gene editing for improving the bioactive molecules in A. triplinervis.
1. INTRODUCTION
Ayapana triplinervis (Vahl) R. M. King & H. Robinson is a widely used folk medicinal plant in the healing processes, and it belongs to the family Asteraceae. The plant is used in mystical-religious rituals by the traditional communities inhabiting Asia, Africa, and South America [1]. It is an erect, perennial, semi-woody at basal region, ornamental herb growing up to a height of 1 m [2]. The leaves of the plant are widely used as decoctions, teas, or baths against viral infections and respiratory and gynecological diseases in Brazil, Bangladesh, Sri Lanka, West Indies, Mauritius, Peru, and some European countries [3]. It is commonly known as 'Ayapana' in India and 'Vishalyakarani' in Ayurvedic literature, and the plant is used in wounds, bleeding stomachs, and diarrhoea because of its capacity to control blood coagulation [3]. The plant is also a good cardiac stimulant, emetic, laxative, expectorant, alterative, and antiscorbutic and is used in cases of dyspepsia and agues [4].
The phytochemical investigations on A. triplinervis by the researchers revealed the presence of secondary metabolites, such as coumarins (ayapin, ayapanin, daphnetin etc.), essential oils (thymoquinone, thymohydroquinone dimethyl ether, β-caryophyllene, β-selinene, dimethyl ether thymoquinone and 2, 5-dimethoxy p-cymene), stigmasterol, and carotene [2, 5]. The pharmacological properties of A. triplinervis have been extensively studied, and the plant was found to possess various biological activities, such as hepatoprotective, antioxidant, antimicrobial, anti-nociceptive, anti-inflammatory, antimelanogenic, anxiolytic, antidepressant, antiulcer, anticancer, hypocholestemic, gastroprotective, and antidiabetic effects [6-16]. A recent study revealed that thymohydroquinone dimethyl ether extracted from the aerial parts of A. triplinervis inhibited Zika virus infection in human epithelial A549 cells [17]. A. triplinervis is one of the most important medicinal plants used in herbal drug development. Ayapin, or 6, 7-methylenedioxycoumarin is a phytoalexin used as an antimicrobial and antiviral agent, was isolated from this plant [18]. The plant contains a variety of coumarins with an anticoagulant activity that are the precursors for drugs, such as 'Warfarin' [19]. The nano-emulsions developed from the essential oils extracted from A. triplinervis are used as mosquito repellant [20, 21].
According to the World Health Organization (WHO), the current demand for medicinal plants is increasing annually, estimated at about 14 billion US dollars [22]. The pharmaceutical industries mainly depend on the wild population of medicinal plants to extract phyto compounds in herbal drug development [23, 24]. However, the commercial management of these phytoconstituents is impeded due to the inadequate supply of medicinal plants [25]. The high demand for medicinal plants motivated rural farmers to start the commercial cultivation of medicinal plants. Previous attempts have been made for the in vitro propagation of A. triplinervis (Synonyms: Eupatorium triplinerve) using different explants. The micropropagation protocol through axillary bud proliferation and ex vitro rooting was reported [26]. Usha and Karpagam [27] developed a regeneration protocol using nodal explants supplemented with 4.44 μM 6-Benzylaminopurine (BAP) and produced 18 shoots with an average length of 6.4 cm. In another study, more shoots (5) with higher shoot length (2.85 cm) were obtained from nodal explants on MS medium fortified with 0.2 mg/L BAP and 0.02 mg/L Gibberellic acid 3 (GA3) after 30 days. In another protocol, MS medium fortified with 17.76 μM/L BAP and 2.69 μM/L of naphthalene acetic acid (NAA) produced 4.93 shoots [28]. These studies showed that the low concentration of phytohormones had poor responses to shoot initiation and multiplication. The optimum concentration of phytohormones could help for better shoot initiation and multiplication. The optimization of phytohormone concentration is essential for the development of an efficient plant regeneration protocol. In this study, the previously reported same plant species A. triplinervis was used to develop an in vitro regenerative protocol. The study describes a rapid regenerative protocol of A. triplinervis using nodal explants for a maximum of 35 shoot induction with the assessment of the genetic fidelity of regenerated plantlets using RAPD markers. This is the first study reporting maximum shoot induction in this plant species. This study may be useful for mass multiplication and biotechnological studies of A. triplinervis.
2. MATERIALS AND METHODS
2.1. Plant Material
A. triplinervis plants were collected from the Rajagiri College of Social Sciences (Autonomous) herbal garden, Kakkanad campus, Kochi, Kerala, India, and a herbarium was prepared for morphological study. Herbarium specimens were taxonomically identified and authenticated by a botanist. Previously reported plant A. triplinervis (Synonyms: Eupatorium triplinerve) was used for this present study [27]. The protocol described by Antony Ceasar et al. [29] was used to prepare nodal explants with minor modifications in the surface sterilization procedure. Nodal explants (approximately 2 cm, each containing two opposite nodes) were washed in sterile water for 15 min and soaked in 0.1% (w/v) Bavistin containing carbendazim (BASF, Mumbai, India) for 20 min. It was then washed three times using sterile distilled water. Surface sterilization was performed by immersing nodal explants in 70% (v/v) alcohol for 10 seconds and washing three times using autoclaved distilled water. After that, the explants were treated with 0.1% (w/v) HgCl2 for 1 min and washed four times using sterile distilled water. The nodal explants were air-dried on filter paper (Whatman No. 1, Code:1001-125, Sigma-Aldrich) in the laminar airflow chamber. The sterilized nodal explants were used for further studies.
2.2. Culture Medium and Conditions
The MS basal medium (Himedia, Code: PT099-25L) was used in the present study [30]. It contained basal salts and vitamins, 3% sucrose (w/v); finally, 0.1 M NaOH was used to adjust the pH 5.6-5.8 of the medium before adding 0.8% agar (w/v) to it. The medium was autoclaved for 15 min at 121°C and 15 lbs. Different concentrations of plant growth regulators were added to the basal MS medium before adding agar. The standardization and shoot induction studies were carried out in test tubes. After that, shoot multiplication, elongation, and root induction tests were performed in tissue culture bottles (6 x 13 cm). All cultures were sub-cultured in the same medium unless otherwise mentioned at 15-day intervals. All cultures were maintained under controlled conditions. The temperature was maintained at 25 ± 2°C and 16/8 light/dark photoperiod using fluorescent tube lights with the light intensity of 50 µmol/m2/s photosynthetic photon flux density (PPFD).
2.3. Shoot Multiplication and Elongation
For shoot induction, nodal explants were cultured on an MS medium supplemented with different plant growth regulators (PGRs), such as BAP and kinetin (KIN). The experiment was performed as part of a standardization procedure to choose the optimum concentration of individual cytokines for efficient induction of shoots. Briefly, different concentrations (10, 20, 30, or 40 mg/L) of BAP and KIN were individually added to the MS medium. Three nodal explants were inoculated in each tissue culture bottle, and three replicates were maintained for each concentration. The optimum individual cytokine concentration was determined based on the number of shoots produced after three weeks of incubation in the light. The percentage of explants that responded and the number of shoots produced by the responding explants were calculated after three weeks of incubation. The three-week-old shoot cultures obtained from various concentrations of shoot induction medium were subcultured in the same multiplication medium for further shoot multiplication and elongation. The MS medium devoid of PGR was considered a control. The mean number of shoots and their length were recorded after four weeks of incubation in the light. The responses of shoot induction were calculated using the following formula [44].
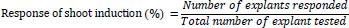 |
2.4. Root Induction
The elongated shoots from the shoot clumps were excised aseptically and transferred to the rooting medium amended individually with 0.5, 1.0, 1.5, or 2.0 mg/L indole-3-acetic acid (IAA) or indolebutyric acid (IBA) for rooting. The total number of roots produced per shoot and the mean length of the roots were measured after four weeks of incubation in the light. The responses of root induction were calculated using the following formula [44].
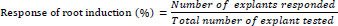 |
2.5. Acclimatization and Hardening
The rooted in vitro plants were cleaned in running tap water before being transplanted into paper cups containing sterilized horticultural-grade perlite (Astra Chemical, Chennai, India) and fed with 1/10 dilution liquid MS medium. The paper cups containing in vitro plantlets were covered with a transparent polythene bag to maintain humidity. These plants were maintained in the culture room for two weeks under the atmospheric conditions of 25 ± 2°C temperature; light 16 h photoperiod with the light intensity of 50 µmol/m2/s PPFD; relative humidity, 85 ± 5%. The 1/10 diluted liquid medium was supplied once in three days. After two weeks, the plantlets were removed from the cup, transferred to the pots containing soil and vermicompost in 1:1 proportion (v/v), and fed with tap water. Again, these plants were kept in the greenhouse for three days. Finally, the acclimatized plants were transferred to the field outside the greenhouse. The regenerated plants were grown ex vivo for about three months, and their survival rate and morphological characteristics were observed.
2.6. Genetic Fidelity Analysis
Genetic fidelity of in vitro regenerated plantlets of A. triplinervis with that of ex-vitro mother plants was confirmed by genomic DNA (gDNA) PCR amplification using random amplified polymorphic DNA (RAPD) primers (Table 1). The gDNA was isolated from in vitro regenerated plants of A. triplinervis (five plantlets were randomly selected) from ex-vitro along with the mother plants. Briefly,gDNA was extracted from a young leaves tissue sample using HiPurA® Plant DNA isolation kit (CTAB method) according to the manufacturer (Himedia, HiGenoMB, Cat. No: MB502-50PR) instructions. The quantity of gDNA was measured using a NanoDrop spectrophotometer (ND-ONE-W, Thermo Fisher Scientific, USA), and the quality of gDNA was checked via a 0.8% (w/v) agarose gel electrophoresis (BIO-RAD, Mini-Sub® Cell GT, USA).
| Sl. No. | Primer Code | Primer Sequence (5' to 3') | Annealing Tm (°C) |
|---|---|---|---|
| 1 | OPE-04 | GTGACATGCC | 40°C |
| 2 | OPF-05 | CCGAATTCCC | 40°C |
| 3 | OPR-07 | ACTGGCCTGA | 40°C |
| 4 | OPG-07 | GAACCTGCGG | 40°C |
| 5 | OPK-10 | GTGCAACGTG | 40°C |
| 6 | OPO-14 | AGCATGGCTC | 40°C |
| 7 | OPW-18 | TTCAGGGCAC | 40°C |
The PCR was set with 25 μL reaction mixture containing 100 ng gDNA, 1X Hi-Chrom PCR master mix, Taq DNA polymerase [1U], dNTPs [200 µM], and MgCl2 [1 mM)]) (Himedia, HiGenoMB, Cat. No: MBT089-50R) and 400 nM RAPD primer. The PCR was performed using a Thermal Cycler (BIO-RAD-T100, USA) with an initial denaturation at 95°C for 5 min, followed by 35 cycles of 30 s denaturation at 95°C, 30 s annealing at 40°C and 1 min extension at 72°C, with a final extension at 72°C for 7 min. The amplicons were resolved in a 2% (w/v) agarose gel electrophoresis (BIO-RAD, Mini-Sub® Cell GT, USA) and stained with ethidium bromide (EtBr). The resolved agarose gels were visualized using the GelDoc Go Imaging system (BIO-RAD, USA).
For data analysis, the amplification pattern of in vitro regenerated plantlets was compared with their mother plant. The amplification products were categorized into four classes, namely: (1) strong band and easy to score (2) weaker band, but able to score; (3) very weak band and challenging to score; and (4) no bands. Classes 1 and 2 were considered positive amplification and taken for the analysis, whereas classes 3 and 4 were considered negative for amplification and excluded from the scoring.
3. RESULTS AND DISCUSSION
3.1. Effect of Cytokinins on Shoot Multiplication and Elongation
Cytokinins are indispensable for developing multiple shoots [31]. They are the critical components of plant tissue culture. This study evaluated nodal explants of A. triplinervis on MS medium with two types of cytokinins (BAP and KIN) at various individual concentrations (10, 20, 30, or 40 mg/L) for developing rapid shoot multiplication protocol. The individual concentration of both BAP and KIN induced the shoot proliferation from the nodal stem segments of A. triplinervis after three weeks of in vitro culture. While MS medium without cytokinins (control) also influenced the growth of nodal segment buds, they did not show any shoot multiplication (Fig. 1). The percentage (%) of shoot induction ranged from 60–90% and 60–100% on MS medium with BAP and KIN, respectively (Table 2). The highest percentage for shoot initiation, 90% and 100%, was observed on MS medium containing 20 mg/L BAP and 20 mg/L KIN, respectively. It was followed by the medium containing 30 mg/L BAP and KIN, which was 80% (Table 2). After the shoot initiation, sub-culturing of the shoot clumps on the respective medium enhanced shoot multiplication (Fig. 2). Eight-week passages on this culture medium resulted in better shoot multiplication and elongation.

(A) Control-MS basal; (B) MS basal containing 20 mg/L KIN and (C) MS basal containing 20 mg/L BAP.

(A) Control-MS basal; (B) MS basal containing 10 mg/L KIN; (C) MS basal containing 20 mg/L KIN; (D) MS basal containing 30 mg/L KIN; and (E) MS basal containing 40 mg/L KIN.
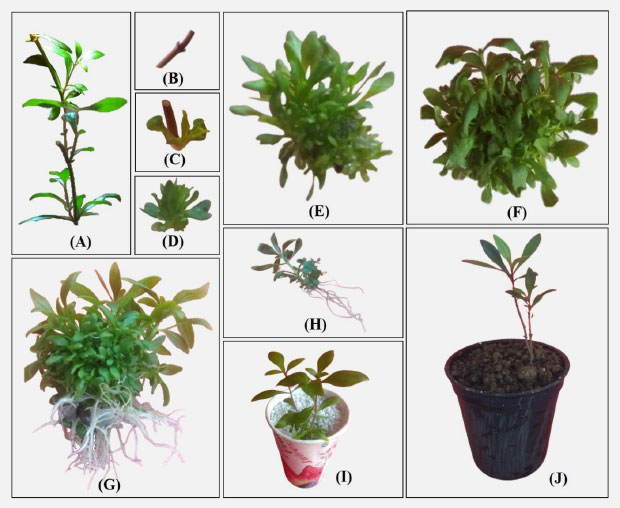
(A) The mother plant of habitat; (B) Nodal bud explants were used for micro-propagation; (C and D) Shoot induction on MS medium containing 20 mg/BAP; (E) Induction of shooting after six weeks under controlled conditions (F) Shoot elongation on MS medium containing 20 mg/L BAP; (G) Elongated shoots inoculated in the MS medium containing 1.0 mg/L IAA; (H) Fully developed plantlets after eight weeks incubation (MS medium + 20 mg/BAP + 1.0 mg/L IAA); (I) Acclimatized plantlets in sterile perlite at lab conditions and (J) Well-established plantlets in the natural environment.
| Growth Regulators (mg/L) | Response of Shoot Initiation (%) | Number of Shoots/Explants | Shoot Length (cm) | |
|---|---|---|---|---|
| KIN | 00 | 00 | 00.00 ± 0.00 | 00.00 ± 0.00 |
| 10 | 70 | 17.00 ± 1.73d | 05.20 ± 0.52b | |
| 20 | 100 | 31.66 ± 2.51a | 06.06 ± 0.30a,b | |
| 30 | 80 | 27.33 ± 1.52b | 07.26 ± 0.41a | |
| 40 | 60 | 20.66 ± 1.15c | 05.50 ± 0.43b | |
| BAP | 00 | 00 | 00.00 ± 0.00 | 00.00 ± 0.00 |
| 10 | 60 | 18.66 ± 1.52d | 06.46 ± 0.41a | |
| 20 | 90 | 35.33 ± 2.08a | 06.50 ± 0.88a | |
| 30 | 80 | 29.33 ± 0.57b | 05.90 ± 0.15a | |
| 40 | 60 | 22.00 ± 2.64c | 04.60 ± 0.35a | |
Among the two cytokinins (BAP and KIN), better shoot multiplication and elongation responses were observed in the medium containing BAP. In BAP supplementation, significantly higher mean shoot number (35.33) and length (6.50 cm) were observed at 20 mg/L BAP (Table 2 and Fig. 3). The medium containing 10 mg/L BAP showed the lowest shoot number (18.66) and length (4.60 cm). Similarly, MS medium supplemented with 20 mg/L KIN showed better shoot multiplication for KIN (Table 2). However, the highest shoot length (7.26 cm) was observed in the medium containing 30 mg/L KIN. Previously, Gandhi and Saravanan [28] analyzed the effect of the combined and individual cytokinins on the shoot initiation and multiplication in A. triplinervis. In particular, the highest shoot number (24) and length (6.50 cm) were observed at 5.15 mg/LBAP and 2.06 mg/L BAP, respectively. The combination of cytokinins (5.15 mg/L BAP + 0.62 mg/L NAA) showed a slight improvement in shoot multiplication (26.00) but did not have significant variations [28]. Therefore, our result showed better shoot multiplication at 20 mg/L BAP with 35 mean shoots per explant.
In contrast with our results, Gandhi and Saravanan [28] reported the highest shoot length (6.50 cm) at the lower concentration of BAP (2.06 mg/L) in A. triplinervis (Synonyms: Eupatorium triplinerve). Our results showed a similar shoot length (6.50 cm) was obtained at 20 mg/L BAP. Also, our results revealed that extremely low (10 mg/L) and high (40 mg/L) concentrations of BAP and KIN inhibited shoot number and length (Table 2). Previous studies indicated that supplementation of higher and lower concentrations of BAP/KIN inhibits shoot multiplication in A. triplinervis [19, 27, 28]. Borchetia et al. [32] observed that higher concentrations of BAP inhibited shoot growth (nodal explant) in tea plants. Supplementation of high concentrations of BAP/KIN could inhibit the adventitious meristem elongation; it may reduce the shoot number [32, 33]. Therefore, the optimum concentration is essential for better and rapid shoot multiplication. Based on our study, a medium containing 20 mg/L BAP helps rapidly multiply A. triplinervis than previously reported works.
3.2. Effect of Auxins on Root Induction
The root is the main organ of plants, and it has many roles and functions. Therefore, adventitious root initiation is the prerequisite for developing a successful tissue culture protocol. Auxins play a leading role in regulating the initial growth of adventitious roots [34]. In this study, the in vitro regenerated A. triplinervis shoots were separated and treated with various concentrations (0.5, 1.0, 1.5, or 2.0 mg/L) of auxins (IAA or IBA) individually for root induction. Root initiation was observed in all concentrations of both IAA and IBA after three weeks of in vitro culture. However, a longer (4-6 weeks) incubation period was needed for better rooting (number and length) of regenerated plantlets. MS medium supplemented with various concentrations of IAA or IBA produced 100% rooting responses (Table 3). The MS medium without auxins (control) also showed root formation in nodal segment buds. However, no root proliferation was shown in the stem (an immersed portion of the MS medium). The findings of the present work supported the results of Balakrishnan et al. [19], who also reported that in vitro raised shoots produced roots in the media used for shoot multiplication without adding auxins. This finding was missing in the previously reported tissue culture on A. triplinervis [28, 35]. Babaei et al. [36] revealed that roots of Curculigo latifolia explant were induced faster in MS medium (control) devoid of plant growth regulators than in various concentrations (0.25, 0.50, or 0.75 mg/L) of IBA. It may be due to sufficient endogenous auxins within the explants. These findings indicated that auxin might be unnecessary for in vitro root proliferation for A. triplinervis.
However, our results revealed that the concentrations of IAA and IBA significantly influenced root initiation. Among the two auxins (IAA and IBA), better initiation was observed with IAA. The MS medium containing 1.0 mg/L IAA showed higher root initiation with 13.66 mean roots per explant (Table 3 and Fig. 3). Similarly, best rooting was observed in MS medium supplemented with 1.0 mg/L IBA with 10.33 mean roots per explant having a mean root length of 4.76 cm (Table 3). In contrast with our results, Samydurai et al. [35] reported that the half-strength MS medium with various concentrations of IAA and IBA (0.2, 0.4, 0.6, 0.8 or 1.0 mg/L) showed the lowest root number and length in A. triplinervis. But, Balakrishnan et al. [19] showed better root initiation (12.36) of A. triplinervis at MS basal with 1.0 mg/L IBA. The present study suggests that 1.0 mg/L IAA was the most suitable concentration for root induction of A. triplinervis.
3.3. Acclimatization and Hardening
The acclimatization of micro-propagated plants is the most precious work because regenerated plantlets are highly susceptible to environmental effects. In this study, the regenerated healthy plantlets were transplanted to an artificial soil mixture (perlite) in paper cups and maintained in a culture room with controlled growth conditions for 14 days for better acclimatization (Fig. 3I). The well-established plantlets were successfully transferred to the ex vitro conditions; they showed good growth and stayed healthy. The regenerated plantlets achieved a 100% survival rate. Their shoots flourished, and leaves became bigger and greenish under field conditions. The plants produced by micropropagation were morphologically and physiologically similar to mother plants. Acclimating in vitro-derived plants to the external environment is crucial for A. triplinervis [27]. Gandhi and Saravanan [28] reported that the in vitro regenerated A. triplinervis plantlets showed a 90% survival rate. However, the present study showed a better acclimatization procedure for successfully surviving in vitro regenerated plantlets.
3.4. Genetic Fidelity Analysis of In Vitro Generated Plantlets
Genetic variations are common and severe in vitro-generated plantlets [37]. Therefore, evaluation of the genetic variation among the in vitro regenerated and mother plants is essential. So, molecular markers are preferable for evaluating genetic fidelity analysis. This study analyzed the genetic fidelity of in vitro regenerated A. triplinervis plantlets and their mother plant using RAPD markers. Totally seven RAPD primers were used in the present study, and these primers generated 40 PCR amplification products ranging from 190 to 1600 bp size (Table 4). The primer OPW 18 amplified the maximum number of nine bands (Fig. 4c) and was followed by primer OPG 07 (Table 4). The OPE 04 and OPF 05 primers amplified the lowest number of bands (Fig. 4a and 4e). Our results showed that all primer banding patterns of the mother plant were similar to in vitro regenerated plants (Fig. 4). No RAPD polymorphism was observed in the in vitro regenerated plants. For example, the primer OPO 14 produced six amplicons ranging from 270-1150 bp (270, 360, 500, 610, 750, and 1150 bp) in the mother and their in vitro generated plantlets of A. triplinervis. These results revealed that the RAPD markers could be an easy way to check the quality of in vitro regenerated plants. Previously, the RAPD markers were used to analyze the genetic homogeneity of micropropagated plants. RAPD marker-based genetic fidelity has been successfully explored in many plants, such as Teucrium polium [38], Citrus limon [39], Gloriosa superba [40], Bacopa monnieri [29], Flemingia macrophylla [41], Andrographis alata [42], and Tecoma stans [43]. To the best of our knowledge, this is the first report on the genetic fidelity analysis of micropropagated plants of A. triplinervis using the RAPD markers. Our results show that RAPD markers can be used to gain rapid and precise information about genetic similarities in vitro generated plants.
| Growth Regulators (mg/L) | Response of Root Initiation (%) | Number of Root/Explant | Root Length (cm) | |
|---|---|---|---|---|
| IAA | 0 | 0 | 00.00 ± 0.00 | 00.00 ± 0.00 |
| 0.5 | 100 | 08.00 ± 2.00c | 03.96 ± 0.25a | |
| 1 | 100 | 13.66 ± 1.15a | 04.36 ± 0.32a | |
| 1.5 | 100 | 12.33 ± 1.52a,b | 03.70 ± 0.25a | |
| 2 | 100 | 12.00 ± 1.73b | 03.40 ± 0.40a | |
| IBA | 0 | 0 | 00.00 ± 0.00 | 0.00 ± 0.00 |
| 0.5 | 100 | 08.66 ± 0.67a,b | 03.53 ± 0.30a | |
| 1 | 100 | 10.33 ± 2.30a | 04.76 ± 0.25a | |
| 1.5 | 100 | 07.00 ± 1.73b | 04.00 ± 0.20a | |
| 2 | 100 | 07.66 ± 0.57b | 03.70 ± 0.23a | |
| Primer Code | No. of the Amplified Band | Size range (bp) | Approximate Size (bp) of the Amplified Band |
|---|---|---|---|
| OPE 04 | 3 | 210-400 | 210, 350 and 400 |
| OPF 05 | 3 | 190-360 | 190, 300, and 360 |
| OPR 07 | 5 | 200-600 | 200, 310,420, 480 and 600 |
| OPG 07 | 8 | 320-780 | 320, 340, 440, 490, 550, 600, 700, and 780 |
| OPK 10 | 6 | 270-1150 | 270, 360, 500, 610, 750 and 1150 |
| OPO 14 | 6 | 330-1160 | 330, 470, 620, 710, 950, and 1160 |
| OPW 18 | 9 | 310-1600 | 310, 350, 430, 480, 580, 650, 690, 950 and 1600 |
| Total | 40 | 190-1600 | - |
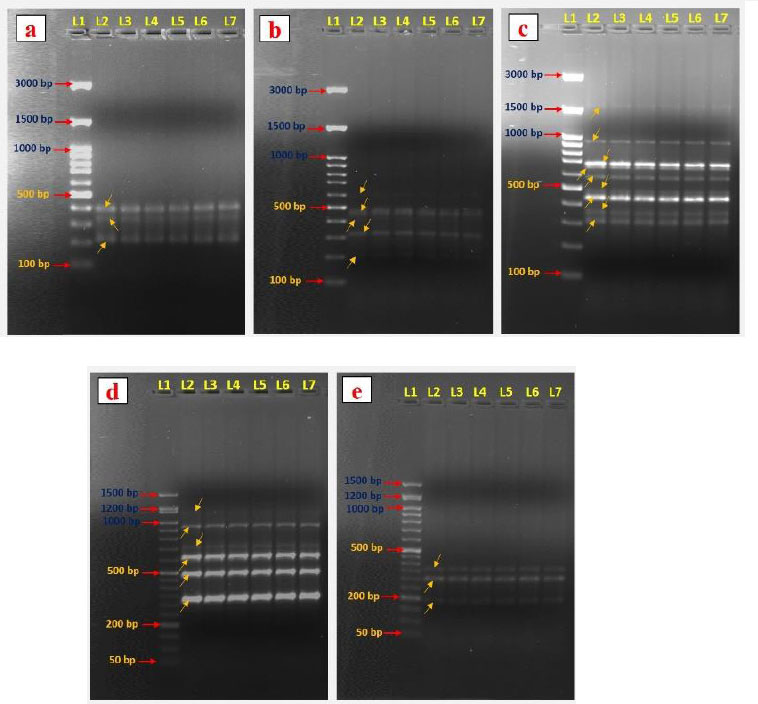
DNA fingerprinting patterns were generated with RAPD primers OPE 04 (a), OPF 07 (b), OPW 18 (c), OPO 14 (d), and OPF 05 (e). Lane 1, DNA markers (100/50 bp ladder); Lane 2, RAPD marker profile of a mother plant. Lanes 3–7, RAPD marker profiles of five separate regenerated plantlets.
CONCLUSION
This study reports an efficient in vitro regeneration protocol for direct shoot regeneration from the nodal explants of A. triplinervis. The MS basal with 20 mg/L BAP showed the highest shoot induction and elongation responses. The better rooting was obtained at 1.0 mg/L IAA. This work reports the optimum concentration of phytohormones for rapid and efficient plant regeneration of A. triplinervis. The acclimatization of in vitro-generated plantlets achieved a 100% survival rate. We successfully determined the genetic fidelity of plantlets using RAPD molecular markers. All seven RAPD primers produced monomorphic bands, confirming the genetic homogeneity of the in vitro-regenerated plants. Moreover, this efficient protocol could benefit future transgenic and gene editing works for altering and improving the biosynthesis pathways for bioactive molecules from A. triplinervis.
LIST OF ABBREVIATIONS
| BAP | = 6-Benzylaminopurine |
| GA3 | = Gibberellic acid 3 |
| IAA | = Indole-3-acetic Acid |
| IBA | = Indolebutyric Acid |
| KIN | = Kinetin |
| MS | = Murashige and Skoog’s |
| NAA | = Naphthalene Acetic Acid |
| PGRs | = Plant Growth Regulators |
| RAPD | = Random Amplified Polymorphic DNA |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used in this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
This work was financially supported by Rajagiri College of Social Sciences (Autonomous), Kalamassery, under Seed Money for Faculty Minor Research to the second author (NMK).
CONFLICT OF INTEREST
Dr. Antony Ceasar Stanislaus is on the Editorial Advisory Board of the journal The Open Biotechnology Journal.
ACKNOWLEDGEMENTS
We sincerely thank Rajagiri College of Social Sciences (Autonomous), Kochi, Kerala, for providing the research facilities and support.


