All published articles of this journal are available on ScienceDirect.
Urtica Dioica Leaf Extract Attenuates Oxidative Stress Induced by Environment Pollutant Benzo[a] Pyrene in Mouse Testicular Tissues
Abstract
Background:
Epidemiological studies have shown that elevated levels of air pollutants are associated with various adverse health effects, including infertility.
Objective:
We aimed to assess the protective effects of aqueous Urtica dioica leaf extract against benzo[a]pyrene -induced oxidative damage in mouse testis.
Methods:
Mice exposed to benzo[a]pyrene were treated with or without aqueous Urtica dioica extract for five weeks, and changes in body and testes weights, messenger RNA levels and activities of antioxidant enzymes, plasma testosterone levels, sperm characteristics, and testicular tissue histology were determined.
Results:
Exposure to benzo[a]pyrene remarkably reduced testis and body weights, the expression and activity of antioxidant enzymes, increased lipid peroxidation, decreased plasma testosterone levels and sperm count and motility, affected sperm morphology and viability, and damaged the seminiferous tubules. Treatment with aqueous Urtica dioica leaf extract attenuated benzo[a]pyrene -induced oxidative stress in the testicular tissue by increasing the expression of antioxidant genes. Further, Urtica dioica leaf extract reduced lipid peroxidation, increased antioxidative enzyme activity, enhanced sperm characteristics, increased plasma testosterone levels, and improved the morphology of the seminiferous tubules.
Conclusion:
Aqueous Urtica dioica leaf extract protects testicular tissue from benzo[a]pyrene -induced oxidative damage and could potentially reverse benzo[a]pyrene -induced infertility.
1. INTRODUCTION
Polycyclic aromatic hydrocarbons (PAH), or polynuclear aromatic hydrocarbons, are organic aromatic hydrocarbons with several benzene ring fusions, comprising more than 100 distinct compounds [1]. They are formed by the incomplete combustion of wood, gas, oil, coal, waste, and other organic substances and are present in cigarette smoke, fumes from grilling food, and vehicle exhaust [2]. Previous studies have reported that PAH can trigger different toxicities, including genotoxicity, oxidative stress, and immunotoxicity [3, 4].
Benzo[a]pyrene (B(a)P) is a widely prevalent PAH. B(a)P is a ubiquitous environmental pollutant with five fused rings. It is found in high concentrations in tobacco smoke, emissions from diesel vehicles, food cooked on charcoal, and industrial residues [5, 6]. Exposure to B(a)P is currently more unavoidable than exposure to other environmental pollutants than ever before [7]. Several studies have reported hazardous consequences of B(a)P exposure [8-10]. The International Agency for Research on Cancer defines B(a)P as a category 2A carcinogen compound due to its deleterious effects on human and animal models [6, 7]. It has been linked to multiple malignancies, including lung, breast, and liver cancer [11-13]. B(a)P is known to cause phenotypic and genotypic abnormalities that last for many generations by inducing cellular and molecular damage by producing reactive oxygen species [7]. Although B(a)P has been designated a carcinogen, it must be metabolically stimulated before it can exert deleterious effects. B(a)P is hydrolyzed into the carcinogenic molecule benzo[a]pyrene-r-7,t-8-dihydrodiol-t9,10-epoxide (BPDE), which directly interacts with DNA after being metabolized by hydrolases in the classic B(a)P metabolic pathway [14]. BPDE, in the presence of other risk factors like ultraviolet light exposure, increases the risk of mutations, cancer, and other negative outcomes [5, 15-17].
When B(a)P is activated, it triggers the production of reactive oxygen species (ROS), which results in oxidative stress. It causes an increase in lipid peroxidation and caspase activity [18]. In addition, B(a)P is genotoxic and epigenotoxic, has an oxidative capacity, and reduces animal fertility [5]. Mutations in reproductive cells can make future generations vulnerable to developmental abnormalities and diseases, such as cancer [19]. Kakavandi et al. studied the relationship between environmental pollutants such as PAHs and male infertility. They reported a significant inverse correlation between the PAH metabolites and several parameters related to sperm characteristics, including count, motility, morphology, and DNA degradation [20]. Another study examined the potential relationship between semen quality, sperm DNA integrity, and working exposure to PAHs using mixture analysis and reported a negative correlation between PAH exposure and sperm vitality [21]. A study on the correlation between heavy cigarette consumption and male infertility found that cigarette smoking elevated BPDE-DNA adducts in the spermatozoa and observed relatively significant quantities in those who did not smoke, revealing that exposure to environmental pollution is also a significant factor that induces the production of BPDE-DNA [22]. The production of BPDE-DNA adducts in sperm cells is a potential source of prezygotic DNA harm. If not corrected, it may result in transmissible cancer-causing mutations [23]. The decrease in sperm production and sperm quality and motility can indicate the physical harm environmental pollutants and smoking can cause male sperm cells [24, 25]. Reducing oxidative stress is crucial for individuals exposed to environmental pollution, as it is widely acknowledged that increased ROS production can result in male infertility [26].
Recent studies have focused on identifying cost-effective botanicals that can improve human health [27, 28]. Due to its beneficial effects on human health, Urtica dioica L. (Urticaceae), often referred to as the stinging nettle, is recognized as a medical herb in many parts of the world [29]. Urticadioica, a member of the Urticaceae family, has been demonstrated to have several pharmacological properties, including antioxidant, anti-inflammatory, anti-proliferative, and anticancer activities [30-32]. Urtica dioica aqueous leaf extracts have been shown to contain trans-caffeate or sodium caffeate, gallate, quercetin, myricetin, gelseminic acid, 1′-OH-gamma-carotene glucoside, secoisolariciresinol, and flavylium [33]. Additionally, it has been demonstrated that U. dioica extract protects the body by functioning as a free-radical inhibitor and source of primary antioxidants [34] and effectively neutralizes ROS [35].
Previous studies have shown that ethanolic and methanolic extracts of U. dioica leaf possess antioxidant properties against 2,2-diphenyl-1-picrylhydrazyl [34]. Previously, an in vivo study investigated the antioxidant and anti-asthmatic effects of the aqueous leaf extract of U. dioica in adult male Wistar rats [36-38]. They evaluated lung, liver, and red blood cell oxidative stress parameters, airway inflammation, and biomarkers of oxidative stress [33]. They observed that treatment with the U. dioica leaf extract effectively inhibited the recruitment of inflammatory cells and substantially reduced lipid peroxidation in the lung tissue [33] and concluded that U. dioica extract had anti-inflammatory properties [33]. In a rat model exposed to carbon tetrachloride (CCl4), U. dioica treatment decreased lipid peroxidation and increased antioxidant activity, preventing hepatotoxicity. This was attributed to phenolic compounds, mostly responsible for this antioxidant capacity [39].
Although several studies have reported the antioxidant capacity of U. dioica L. (Urticaceae), there is a paucity of literature on the effects of its aqueous leaf extract on chemically induced oxidative stress. In an effort to address this gap of knowledge, this study aimed to explore the antioxidant properties of aqueous U. dioica L. (Urticaceae) leaf extract against B(a)P-induced oxidative injury in the testicles of Swiss albino mice. Specifically, this study investigated whether and how U. dioica L. aqueous extract restores the antioxidant gene expression, antioxidant enzymes, and plasma testosterone level and improves the spermatological parameters and histopathological indexes in testicular tissue exposed to B(a)P.
2. MATERIALS AND METHODS
2.1. Preparation of Aqueous U. Dioica Leaf Extract
Fresh U. dioica leaves were collected in January 2023 from Al Hada Heights in the Taif Governorate and authenticated by the Biotechnology Department of the School of Science, University of Taif. The leaves were cleaned, shade-dried, and ground into a fine powder. Next, 1 g of dried U. dioica leaves was soaked in 15 mL distilled water and incubated for 4 h in a water bath at 65 °C. The extract was filtered through Whatman No. 1 filter paper and stored in the dark at 4 °C until use [40].
2.2. Benzo[a]pyrene (B(a)P)
Analytical grade B(a)P was purchased from Sigma Aldrich (St. Louis, MO, USA) (Molecular formula: C20H12, Molecular weight: 252.31 g/mol, concentration ≤ 100%).
2.3. Experimental Animals and Ethical Approval
Adult Swiss male albino mice (12 weeks old, average weight = 30 g) were purchased from the Animal House at the King Fahd Center for Medical Research (King Abdulaziz University, Jeddah, Saudi Arabia). The animals were housed in polypropylene cages at 23 ± 2 °C and a 12-h light/dark cycle, with access to a standard diet and water ad libitum. All experimental protocols were approved by the Scientific Research Ethics Committee of Taif University, Taif (No. 44-261). Throughout the procedure, precautions were taken to reduce pain. The animals were acclimatized for seven days before the experiment.
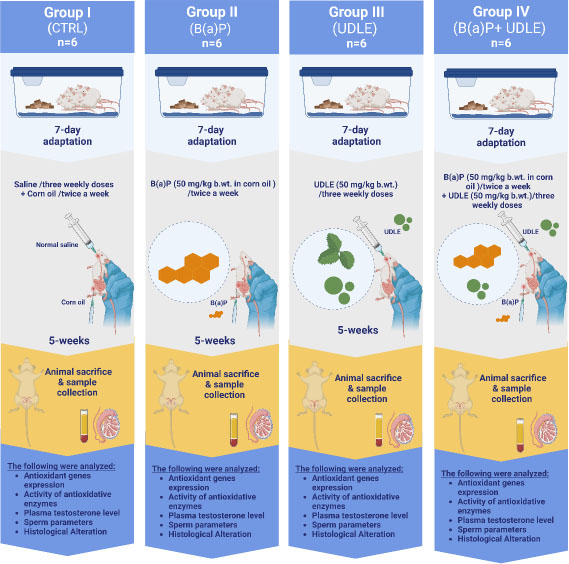
2.4. Experimental Animal Grouping and Dosing
The dosages of B(a)P and aqueous U. dioica leaf extract were determined based on previous studies [41, 42]. The mice were randomly divided into four groups of six, with each group housed in a separate cage.
Group I: The control group (CTRL) received three weekly doses of normal saline (10 mL/kg of body weight [b.wt]) by intraperitoneal administration of corn oil (10 mL/kg b.wt) via intraperitoneal administration twice weekly for 5 weeks.
Group II: Benzo[a]pyrene group (B(a)P) received B(a)P (50 mg/kg b.wt in corn oil) by intraperitoneal administration twice weekly), for 5 weeks.
Group III: Urtica dioica group (UDLE) received three weekly doses of aqueous U. dioica leaf extract (50 mg/kg b.wt) by intragastric administration for 5 weeks.
Group IV: Benzo[a]pyrene and U. dioica (B(a)P+ UDLE) group received three weekly doses of aqueous U. dioica leaf extract (50 mg/kg b.wt.) by intragastric administration and B(a)P (50 mg/kg body weight) by intraperitoneal administration twice a week for 5 weeks. Aqueous U. dioica leaf extract was administered on Sundays, Tuesdays, and Thursdays, and B(a)P on Mondays and Wednesdays.
During the study period, the body weight and health of the animals were monitored and recorded weekly. After five weeks, the body weights of all animals were measured, the mice were sacrificed by cervical decapitation. Serum was collected after drawing blood from the retroorbital sinus for plasma testosterone analysis. Testicular tissue samples were quickly removed and weighed for RNA extraction and other examinations, including biochemical and histological analyses. Fig. (1) illustrates the experimental design.
2.5. Quantification of Antioxidant Gene mRNA Expression Levels
The High Pure RNA Tissue Kit (Roche, Basel, Switzerland) was used to extract total RNA from testicular tissues, following the manufacturer's instructions. Total RNA was quantified using the Jenway Genova Nano Microvolume Spectrophotometer (Fisher Scientific International, Inc., Hampton, NH, USA). First-strand cDNA was synthesized using the ProtoScript First-Strand cDNA Synthesis Kit (New England Biolabs, Ipswich, MA, USA) following the manufacturer's standard protocol. Luna® Universal qPCR Master Mix (New England Biolabs) was used to perform qPCR following the manufacturer's standard protocol using 10 µM from the forward and reverse specific primers. The sequences of the specific primers were as follows: glutathione peroxidase 1 (GPx-1) (Forward) 5′- GTCCACCGTGTATGCCTTCT -3′ and (reverse) 5′- CTCCTGGTGTCCGAACTGAT -3′; superoxide dismutase 1 (Sod-1) Forward) 5′- GAGACCTGGGCAATGTGACT -3′ and (reverse) 5′- GTTTACTGCGCAATCCCAAT -3′; catalase (CAT) (Forward) 5′- ATGGATTCTCCCACTCCGGA -3′ and (reverse) 5′- ACCGCACAGCACAGGAATAA -3′; Beta-actin (Forward) 5′- AGCCATGTACGTAGCCATCC -3′ and (reverse) 5′- CTCTCAGCTGTGGTGGTGAA -3’. The cycling program included initial denaturation at 95 °C for 60 s (1 cycle) followed by 40 cycles of 95 °C for 15 s denaturation and 60 °C for 45 s for annealing/extension. Target genes within each sample were normalized to Beta-actin (Actb) [43]. The mean ± Standard deviation (SD) from three replicates is shown in the results.
2.6. Biochemical Evaluation
Testicular tissue samples from each group were washed three times with 1× PBS and homogenized in tissue homogenization buffer (0.01 M Tris-HCL) using a glass Teflon® pestle homogenizer. The homogenate was subsequently separated by centrifugation at 8500 × g for 10 min at 4 °C. The supernatant (homogenate) was used to evaluate lipid peroxidation (LPO) and the activities of superoxide dismutase (SOD), glutathione S-transferase (GST), and glutathione peroxidase (GPx). For LPO analysis, the protocol described by Muralidhara and Narasimhamurthy was performed [44] using thiobarbituric acid (TBA) (Sigma, St. Louis, MO, USA). The method described by Kakkar et al. was used to measure superoxide dismutase (SOD) activity [45]. GST activity was analyzed as previously described [46]. Briefly, 200 µL of the homogenate was mixed with 50 µL of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) substances (Sigma, St. Louis, MO, USA) and 1720 µL potassium phosphate buffer, and then the absorbance at 412 nm was measured using the Evolution™ 201/220 UV-Visible Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The method described by Rotruck et al. was used to determine glutathione peroxidase (GPx) activity [47]. The mean ± Standard deviation (SD) from three replicates is shown in the results.
2.7. Plasma Testosterone Level
The plasma testosterone level in each group was measured using the Elecsys Testosterone II assay (Cobas ®, Roche, Basel, Switzerland) following the manufacturer’s instruction, using a Cobas e 601 analyzer (Roshe). The mean ± SD plasma testosterone levels from three replicates are shown in the results.
2.8. Spermatological Examination
The right cauda epididymis from the mice in all groups was carefully collected and rinsed in prewarmed Hanks' Balanced Salt Solution (HBSS) (Sigma Aldrich) at 37 °C. The epididymis was placed in a prewarmed petri dish, 1.5 ml of prewarmed HBSS was added, and the epididymis was chopped into small sections and incubated for 20–25 min at 37 °C with gentle agitation to release sperm into the medium. Sperm samples were incubated for 20–25 min at 37 °C, and sperm parameters were analyzed under a light microscope at a magnification of 400 × following the laboratory manual of the World Health Organization (WHO) [48].
To assess sperm count, 1 mL sperm suspension was mixed with 1 mL 10% formaldehyde fixative. Approximately 10 µL diluted sperm suspension was placed on a hemocytometer Malassez Counting Chamber (Fisher Scientific, UK), and the number of sperms in the four small corner and center squares was counted under a phase contrast microscope. The mean ± SD sperm counts from three replicates are shown in the results.
For measuring the percentage of motile sperm, approximately 5 µL semen suspension was placed on a slide and video recorded for 20 s at a magnification of 400x. ; ten microscopic fields from each slide were used to enumerate motile and immotile sperm. The mean ± SD sperm motility from three replicates is shown in the results.
The percentage of viable sperm was determined by mixing 20 µL sperm suspension with 7 µL of trypan blue to stain the sperm and observed under a light microscope at a magnification of 400x. The unstained sperm were deemed viable, and the stained ones were deemed dead. Percentage sperm viability was determined after counting at least 500 sperm from each sample in ten microscopic fields. The mean ± SD sperm viability from three replicates is shown in the results.
The sperm suspension was centrifuged for 5 min at 400 × g, the supernatant was discarded, and 3 mL 1× PBS was added, mixed gently, centrifuged for 5 min at 400 × g, and the supernatant was discarded. The pellet was dissolved in 200 µL 1 × PBS; 10 µL of the suspension was placed at the base of a clean slide and smeared with another slide. The slide was dried at 25 °C, immersed for 1 h in 75% ethanol, and dried at 25 °C. The slide was stained in 0.4% eosin solution (Sigma Aldrich) for 1 h and dried at 25 °C. The sperm morphology was evaluated and photographed under a bright-field light microscope at 400 × magnification. A minimum of 1000 sperms were examined to differentiate spermatozoa based on their typical or atypical head and tail morphology [49]. The mean ± SD sperm abnormality from three replicates is shown in the results.
2.9. Histopathological Investigation
Testicular tissue was collected from mice from all experimental groups and fixed in 10% formalin (Sigma) for 24 h. The samples were then washed and dehydrated in increasing concentrations (70%, 90%, and 100%) of ethanol, embedded in paraffin using a Tissue-Tek TEC 5 embedding console system (Sakura Finetek, USA, Inc. Torrance, CA) and 4 μm-sections were cut using the Accu-Cut® SRM™ 200 Rotary Microtome (Sakura Finetek USA, Inc.). Each slice was placed in a hot water bath at 45 °C, collected on a slide, and placed in an oven at 57.7 °C for 15 min. Slides were placed in the Leica Autostainer XL ST5010 (Richmond Scientific Ltd., Chorley, England) for hematoxylin and eosin (H&E) staining and examined under a light microscope at 100 × and 200 ×. Images were captured with an OMAX TM A35180u3 microscope digital camera and OMAX toupView version 4.11(OMAX TM, Kent, WA, USA). Representative images from three replicates are shown in the results.
2.10. Data Analysis
Statistical analyses were performed using GraphPad Prism version 9.5.1. Two-way analysis of variance ANOVA was used to assess the differences in body weight changes between experimental groups. One-way (ANOVA) was used to assess the differences between the experimental groups in testis weight changes, gene expression, biochemical analysis, and sperm parameters. The results are expressed as mean ± standard deviation (SD). Multiple comparisons were used to compare the means of groups II (BPDE), III (UDLE), and IV (B(a)P+ UDLE) with those of the control group (I) and the mean of Group II with Group IV. P˂ 0.05 was deemed statistically significant.
3. RESULTS
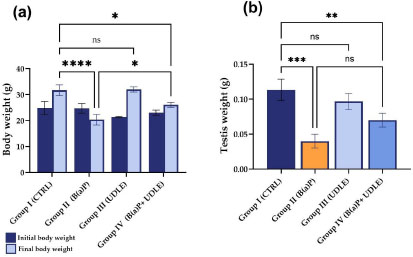
3.1. Urtica Dioica Leaf Extract Attenuated the B(a)P-induced Decrease in Body and Testicular Weight
Mice treated with B(a)P (Group II) showed a significant decrease in body weight compared to that of the control (Group I) (P < 0.0001), while mice treated with the aqueous U. dioica leaf extract (Group III) did not show any statistically significant changes in body weight compared to the control (Fig. 2a). Mice that were exposed to B(a)P and treated with the aqueous U. dioica leaf extract (Group IV) showed a significant reduction (P < 0.05) in final body weight compared to the control (Fig. 2a). However, administration of the aqueous U. dioica leaf extract attenuated the B(a)P-induced decrease in body weight (P < 0.05) (Fig. 2a).
Similar to the trend in body weight, mice treated with B(a)P alone (Group II) showed a significant decrease in testis weight compared to control (Group I) (P < 0.001) (Fig. 2b), while those treated with aqueous U. dioica leaf extract alone (Group III) did not show any significant changes in testis weight compared to the control. Mice exposed to B(a)P and treated with aqueous U. dioica leaf extract (Group IV) showed a significant decrease in testis weight compared with the control (Fig. 2b). However, compared to Group II, Group IV mice showed higher testis weight, albeit not statistically significant (Fig. 2b), suggesting that treatment with aqueous U. dioica leaf extract mitigated the effect of B(a)P on body and testis weight loss.
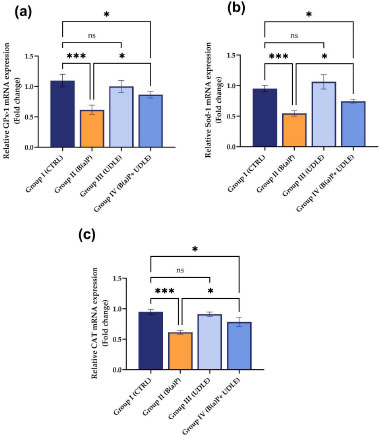
3.2. Urtica Dioica Leaf Extract Attenuated the B(a)P-induced Decrease in the Expression of Antioxidant Genes
Alterations in the expression levels of GPx-1, Sod-1, and CAT genes were assessed in the testicular tissues of mice treated with B(a)P alone, U. dioica leaf extract alone, or both B(a)P and U. dioica leaf extract. A significant downregulation (P < 0.001) in the expression levels of GPx-1 (Fig. 3a), Sod-1 (Fig. 3b), and CAT (Fig. 3c) was observed in mice treated with B(a)P alone (Group II) compared to the control group, while those treated with U. dioica leaf extract alone (Group III) did not show any alterations the expression levels. Mice that were exposed to B(a)P and treated with U. dioica leaf extract (Group IV) showed a significant increase (P < 0.05) in GPx-1 (Fig. 3a), Sod-1 (Fig. 3b), and CAT (Fig. 3c) expression compared to those treated with B(a)P alone (Group II).
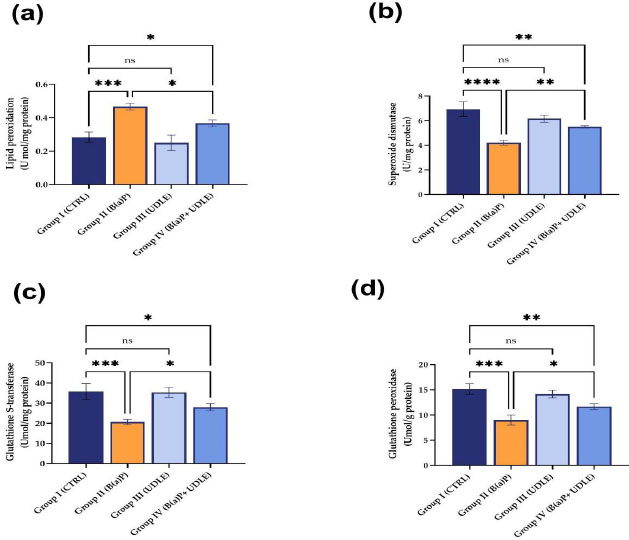
3.3. Urtica Dioica Leaf Extract Reversed the B(a)P-induced Increase in Lipid Peroxidation and Attenuated the Decrease in the Activities of Antioxidant Enzymes
Mice exposed to B(a)P alone (Fig. 4a) showed increased lipid peroxidation compared to the control (Group I). Treatment with U. dioica leaf alone extract did not affect lipid peroxidation. Although treatment with U. dioica leaf extract attenuated the increase in lipid peroxidation induced by B(a)P (Fig. 4a), the level of lipid peroxidation in mice that were exposed to B(a)P and treated with U. dioica leaf extract was still significantly higher than that in the control (Fig. 4a).
Exposure to B(a)P significantly decreased the activities of superoxide dismutase (Fig. 4b), GST (Fig. 4c), and glutathione peroxidase (Fig. 4d) compared to the control group. Treatment with U. dioica leaf extract attenuated the B(a)P-induced decrease in the activity of superoxide dismutase (Fig. 4b), GST (Fig. 4c), and glutathione peroxidase (Fig. 4d). These suggested that U. dioica leaf extract protected the mice from B(a) P-induced toxicity in mouse testis.
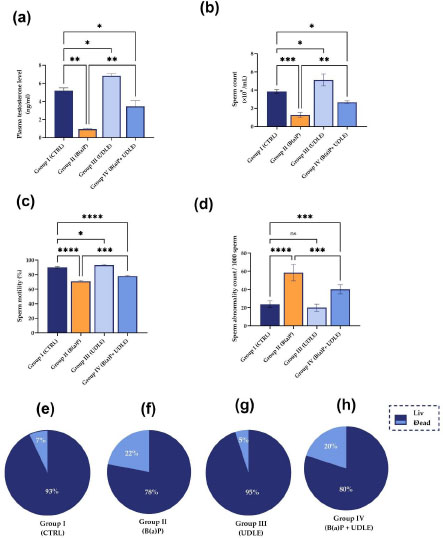
3.4. Urtica Dioica Leaf Extract Improved Testosterone Levels and Sperm Characteristics in B(a)P-exposed Mice
Treatment with B(a)P (Group II) significantly reduced (P < 0.01) and treatment with U. dioica leaf extract (Group III) significantly increased the plasma testosterone levels in contrast to the control group (P < 0.05) (Fig. 5a). Mice exposed to B(a)P and treated with U. dioica leaf extract (Group IV) showed a significant increase in plasma testosterone levels compared to Group II (P < 0.01); however, the levels were significantly lower than that in control (P < 0.05) (Fig. 5a).
In line with the trend in testosterone levels, mice treated with B(a)P (Groupd II) showed a significant decrease in sperm count (P < 0.001) (Fig. 5b) and sperm mobility (P < 0.0001) (Fig. 5c), and a significant increase in sperm abnormality count (P < 0.0001) (Fig. 5d) compared to the control (Group I). However, mice treated with aqueous U. dioica leaf extract alone (Group III) showed a significant increase in sperm count (P < 0.05) (Fig. 5b) and sperm motility (P < 0.05) (Fig. 5c) compared to the control. Moreover, treatment with aqueous U. dioica leaf extract did not affect the morphological properties of the sperm (Fig. 5d). Mice exposed to B(a)P and treated with aqueous U. dioica leaf extract (Group IV) had significantly higher sperm count (P < 0.01) (Fig. 5b) and sperm mobility (P<0.001) (Fig. 5c) compared to those treated with B(a)P alone (Group II); however, these values were still significantly lower than that of the control. Similarly, mice exposed to B(a)P and treated with aqueous U. dioica leaf extract (Group IV) showed a significant decrease in sperm abnormalities compared to those treated with B(a)P alone (P < 0.001) (Fig. 5d), albeit they had significantly a higher abnormality count (P<0.001) compared to the control.
Sperm viability in the control mice (Group I) reached 93% (Fig. 5e), whereas mice treated with B(a)P (Group II) showed a decrease in sperm viability (78%) (Fig. 5f). Mice treated with aqueous U. dioica leaf extract alone (Group III) showed sperm viability of 95% (Fig. 5g) (Group I). The sperm viability in the mice treated with both B(a)P and aqueous U. dioica leaf extract (Group IV) reached 80% (Fig. 5h), which indicated the positive effect of the aqueous U. dioica leaf extract in improving the vitality of the sperm.
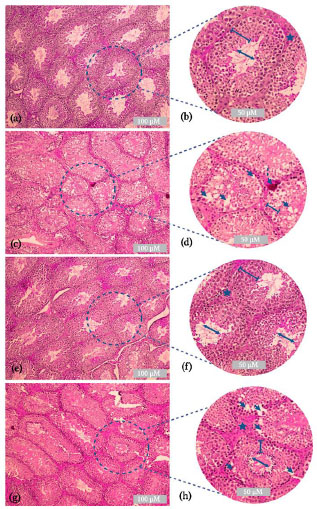
3.5. Urtica Dioica Leaf Extract Attenuated the B(a)P-induced Damages to the Testicular Tissue
The control group (Fig. 6a-b) showed normal morphological features of the testicular tissue sections; the seminiferous tubules were nearly intact and well-organized. The seminiferous tubules were surrounded by myeloid cells. The germinal epithelium of the tubules was intact and contained Sertoli cells and spermatocytes at various phases of development (lines). The lumen was narrow in each tubule (double arrows). The Leydig cells showed a compact arrangement (star) and normal blood vessels. B(a)P treatment induced disorganization and damage to the seminiferous tubules. Intraepithelial vacuolation was observed in the germinal epithelium (arrow). Seminiferous tubules were observed with spermatocytes arrested in the spermatogenic phase (lines). Some congestion of the blood vessels was observed (dashed arrows) (Fig. 6c-d). Mice treated with aqueous U. dioica leaf extract showed similar morphological features to those of the control group (Fig. 6e-f). Intact germinal epithelium of the tubules containing Sertoli cells and spermatocytes was observed at various phases of development (line). A narrow lumen was observed in each seminiferous tubule (double arrow). Intact Leydig cells were recorded (stars). Mice exposed to B(a)P and treated with U. dioica leaf extract showed a moderate improvement with intact germinal epithelium and spermatocytes at various phases of development (line). A narrow lumen was found in many seminiferous tubules (double arrows). A compact arrangement of several Leydig cells was observed (star). Abnormal morphological features, such as intraepithelial vacuolation, were observed (arrow) (Fig. 6g-h).
4. DISCUSSION
The present study evaluated the extent to which aqueous U. dioica leaf extract protected testicular tissues against B(a)P in Swiss albino mice. Our results revealed that after five weeks of exposure to B(a)P, body weight and testis weight were significantly reduced, as has been previously shown to be a typical adverse effect of B(a)P exposure [50, 51]. Co-treatment with aqueous U. dioica leaf extract significantly improved the B(a)P-induced reduction in body and testis weights. In addition, B(a)P treatment significantly downregulated the expression of GPx-1, Sod-1, and CAT genes, whereas animals exposed to B(a)P and co-treated with aqueous U. dioica leaf extract exhibited a significant attenuation of the B(a)P-induced decrease in expression of these genes. A dramatic increase in lipid peroxidation and decreases in the activity of antioxidant enzymes, including superoxide dismutase, GST, and glutathione peroxidase, were observed in testicular tissues in animals treated with B(a)P. Co-treatment with aqueous U. dioica leaf extract rescued the B(a)P-induced changes in lipid peroxidation and antioxidant enzyme activities. Co-treatment with U. dioica aqueous leaf extract substantially reduced the oxidative damage induced by B(a)P.
Epidemiological studies have shown that elevated levels of air pollutants are linked to various adverse health effects, including infertility [18]. B(a)P is a prominent carcinogenic chemical sequentially activated by metabolic reactions, primarily by the cytochrome P450 superfamily enzymes, to produce the highly reactive product BPDE [51]. Because of its extreme carcinogenic and mutagenesis potential, BPDE is often considered the most potent carcinogenic metabolite that can generate multiple DNA adducts [52-54]. Therefore, the International Agency for Research on Cancer classified B(a)P as a carcinogen to humans [55]. Several studies have indicated that B(a)P metabolic activation in the body causes mutagenic and carcinogenic effects on DNA [51, 56, 57]. Intracellular B(a)P activity stimulates several molecular mechanisms, including ROS generation, such as changes in the levels of hydrogen peroxide (H2O2), superoxide anion radicals (O2•–), and hydroxyl radicals (•OH) [58]. The generation of ROS can be harmful and compromise the integrity of cells and tissues [59]. Oxidative stress causes DNA and RNA damage in sperm and is a common primary cause of male sterility [61, 59]. Elevated cellular oxidative stress is significantly associated with male infertility [61]. Recent studies have indicated that a diet rich in flavonoids possesses antioxidant properties in vivo and in vitro [61-63].
Antioxidant enzymes help prevent the harmful effects of oxygen-derived species generated during normal cellular metabolic processes or oxidative stress [64]. Antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and GST, are essential for preventing ROS-induced damage to proteins and DNA, which can trigger apoptosis and other cell death pathways [65]. The current study showed a significant increase in lipid peroxidation levels and a reduction in the activity of antioxidative enzymes in animals administered B(a)P. Co-exposure to aqueous U. dioica leaf extract significantly lowered the level of lipid peroxidation and increased the activity of antioxidative enzymes in the testicular tissues of mice. These results demonstrate that aqueous U. dioica leaf extract suppresses lipid peroxidation and significantly increases the activity of antioxidant enzymes, thus preventing oxidative stress. This suggests the antioxidant ability of aqueous U. dioica leaf extract in minimizing B(a)P-induced oxidative stress. Several male reproductive system-targeting plant extracts in contemporary medicine have been reported recently [66-68].
Urtica dioica L is abundant in flavonol myricetin [69]. Numerous studies have evaluated the effect of flavanols on B(a)P-induced cytotoxicity [41, 70, 71]. The ability of myricetin to prevent the cytotoxicity of B(a)P was investigated in HepG2 cells, and it was observed that combined administration of myricetin with B(a)P decreased the generation of the BPDE-DNA adduct and restored cell viability [72]. The protective role of myricetin in preventing genotoxicity in Sprague–Dawley male rats after exposure to B(a)P was also investigated, and it was found that myricetin inhibited the formation of BPDE-DNA adducts and 8-hydroxy-2′-deoxyguanosine (8-OHdG) in many tissues, such as the stomach, liver, colon, and kidneys [72]. Furthermore, it was reported that myricetin controlled phase I and II enzymes by downregulating the expression of Cyp1a1 and upregulating the expression of GST, which is responsible for blocking B(a)P metabolism and the production of the DNA-conjugated metabolite of B(a)P [72]. The suggested molecular mechanism of the effect of aqueous U. dioica leaf extract on B(a)P-induced oxidative stress in the testicular tissue is shown in Fig. (7).
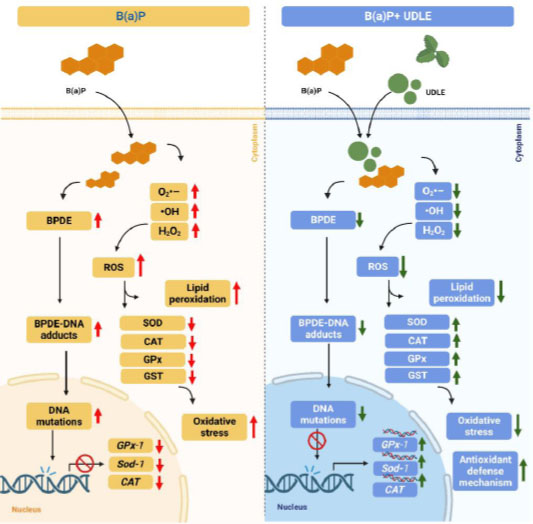
A previous study found that intraperitoneal injection of B)a(P to seven-day-old embryos resulted in aberrant neural tube development in an Institute of Cancer Research (ICR) mouse model. This is because treatment with B(a)P significantly decreases Gpx1 expression and significantly increases the expression level of Sod1, Sod2, and Cyp1a1 [18, 72]. It was shown previously that B(a)P triggered the activation of NF-κB and reduced the production of antioxidative enzymes, including glutathione reductase, glutathione peroxidase, superoxide dismutase, and catalase [73].
In the present study, U. dioica co-treatment remarkably attenuated the effect of B(a)P on testicular tissues, which could be due to its ability to remove •OH, O2•–, and H2O2 radicals by interacting with them. This demonstrates the potential of aqueous U. dioica leaf extract to prevent oxidative injury in testicular tissues by elevating the expression and protein levels of antioxidants.
In the current study, testosterone levels, sperm count, viability, motility, and normal morphology were significantly enhanced in animals treated with aqueous U. dioica leaf extract. The aqueous U. dioica leaf extract enhances the above parameters by stimulating antioxidant defense mechanisms [74]. It has been shown that by upregulating the expression of antioxidant genes, U. dioica functions as an antioxidant and enhances sperm cell quality and quantity [75].
In the current study, the histological examination of testicular tissue sections from animals treated with B(a)P showed disorganization and damage to the seminiferous tubules, which could be due to the production of ROS, resulting in increased lipid peroxidation and decreased antioxidant activity. The formation of morphologically and physiologically fully mature sperms may be disrupted by mitochondrial DNA mutations caused by high ROS levels [76]. Therefore, germ cells can ultimately undergo structural injury and malfunction because of this process [76]. Mice co-treated with aqueous U. dioica leaf extract showed improvement and recovery of several parameters in testicular tissues with abnormal morphological features. This suggests that aqueous extract of U. dioica leaves can recover multiple characteristics during spermatogenesis and the development of mature sperm cells. The obtained results are consistent with earlier published research that proved the ability of U. dioica L. to reduce histopathological changes in testicular tissues of BALB/c male mice treated with nicotine as a source of oxidative stress and a possible cause for decreased fertility in males, suggesting that U. dioica extract supplementation could improve the integrity of sperm and reduce the negative effects of nicotine on seminal parameters [77].
CONCLUSION
The current study demonstrated that co-administration of aqueous U. dioica leaf extract protects testicular tissues from oxidative damage induced by B(a)P by lowering the level of lipid peroxidation and increasing the activity of antioxidative enzymes. Observing antioxidant gene expression data in testicular tissues aligns with the biochemical, spermatological, and histological findings. The results of this study indicate that the aqueous extract of U. dioica leaves has antioxidant activity. These results suggest that the aqueous extract of U. dioica leaves could be developed as a therapeutic drug in the clinic and that identifying and purifying the active compounds could result in the development of novel drugs for use as an antioxidant dietary supplement to reduce infertility and enhance fertility in humans. In addition, further investigations are needed to gain a deeper understanding of the molecular pathways involved in the decreased fertility and changes in the testicular tissue after exposure to B(a)P.
LIST OF ABBREVIATIONS
| ANOVA | = Analysis of variance |
| B(a)P | = Benzo[a]pyrene |
| BPDE | = Benzo[a]pyrene-r-7t-8-dihydrodiol-t9,10-epoxide |
| CAT | = Catalase |
| CTRL | = Control |
| DTNB 5 | = 5′-dithiobis-(2-nitrobenzoic acid) |
| GST | = Glutathione S-transferase |
| H&E | = Hematoxylin and eosin |
| HBSS | = Hanks' Balanced Salt Solution |
| LPO | = Lipid peroxidation |
| PAH | = Polycyclic aromatic hydrocarbons |
| ROS | = Reactive oxygen species |
| SD | = Standard deviation |
| SOD | = Superoxide dismutase |
| UDLE | = Urtica dioica group |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All animal experimental protocols were approved by the Scientific Research Ethics Committee of Taif University, Taif (No. 44-261).
HUMAN AND ANIMAL RIGHTS
No humans were used for studies that are the basis of this research. All the animal experimentation was performed according to the “Guide for the Care and Use of Laboratory Animals.”
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [S.A], on special request.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


