All published articles of this journal are available on ScienceDirect.
Effect of Steeping Duration and Inoculum Concentration on Growth Promotion of Seedlings of Food Crops Primed in Cultures of Bacillus spp from Rhizosphere
Abstract
Aims:
Seed priming represents a viable, low-cost approach to improving germination and plant growth. This study aimed to assess the effects of steeping duration and inoculum concentration of seed crops on germinability enhancement of seed primed in cultures of Bacillus species from rhizospheres. The seeds used were cowpea (Vigna unguiculata), soya bean (Glycine max), sorghum (Sorghum bicolor), sesame (Sesamum indicum), and okra (Abelmoschus esculentus).
Methods:
A total of five Bacillus species (four species of B. cereus and one species of B. thuringiensis) were used for priming the seeds. For the effect of steeping duration on germinability, viable surface-sterilized seeds were primed in growth broth cultures of the respective isolates. Every one hour, for a five-hour duration, a known number of seeds were withdrawn from the cultures and sown. In the case of initial inoculum, seeds were steeped in different dilutions of the bacterial cultures at the optimal steeping duration obtained in the first study before planting. At the expiration of planting duration, final germination percentage, germination time, germination index, and seedling vigor index of the respective seeds were estimated.
Results:
The results highlight the importance of steeping duration for seeds such as cowpea and soybean, and the effect of inoculum concentration was less drastic than that of steeping duration.
Conlcusion:
Further field studies need to be carried out to validate these results, using results here as baseline data.
1. INTRODUCTION
Currently, plant productivity in agricultural production is heavily reliant on the use of synthetic agrochemicals. Backer et al. [1] posited that a new agricultural revolution is needed to meet the nutritional needs of a growing world population, especially in the face of the huge environmental cost associated with the use of agrochemicals. In line with this mantra of environmental sustainability, La Torre-Ruiz et al. [2] and Gamez et al. [3] proposed the use of nutrient-solubilizing PGPB (plant growth-promoting bacteria) as substitutes to help reduce the dependence on synthetic agrochemicals. Microorganisms actively involved in crop production are generally termed plant growth-promoting bacteria (PGPB) [4].
Plant growth-promoting bacteria (PGPB) refers to soil bacteria that occur freely in the soil or in close association with plant roots and have beneficial effects on crop productivity [5]. Plant growth-promoting bacteria (PGPB) are known to promote plant growth and health under various environmental conditions [2, 6-8]. They possess the ability to regulate hormones and metabolic processes that can help prevent the adverse effects of environmental stress. Mechanisms of plant growth promotion include but are not limited to nitrogen fixation, phosphate solubilization, siderophore and phytochemical production, and production of volatile growth enhancement chemicals [9-11].
McDonald [12] defined seed priming as the steeping of seeds in solutions containing the priming agent of interest to allow for the initiation of the germination process but not actual germination, followed by seed drying. It can be used to enhance seed germination capacity under a variety of environmental conditions [13, 14]. Seed priming can also promote seedling development [13].
Common methods of seed priming include hydropriming (with water), osmopriming (with mannitol, polyethylene glycol, and glycerol), hormonal priming (with gibberellins, salicylic acid, and ascorbic acid), biopriming (with microorganisms or biological compounds), and solid-matrix priming (with insoluble matrices, such as sand, vermiculite, peat, or charcoal) [14]. Seed priming can enhance seed performance, particularly concerning germination rate and uniformity, thereby promoting better seedling stand and crop development [15]. However, no priming technique is effective across various crop species, and varying degrees of effectiveness may be observed between varieties of a particular crop [14, 16].
Biopriming entails hydration with the use of biological agents [17]. Seed biopriming results in bacterial colonization of seeds [18] and improves rhizosphere colonization, and decreases plant susceptibility to stress, diseases, and adverse environmental conditions [19]. Steeping of seeds in bacterial suspensions kick-starts physiological processes that enhance germination [20]. Biopriming using rhizosphere bacteria has been applied on several crops [21-25].
Beneficial bacterial strains can enhance nutrient supply, secrete plant growth regulators [1, 26], solubilize important nutrients [27, 28] or inhibit plant pathogens and increase resistance to stress conditions [29]. Saleem et al. [30] stated that these benefits are in tandem with the principles of sustainable agriculture.
Therefore, given the established efficacy of microorganisms as bio-primers, there is a need for proper calibration of priming parameters for effective and efficient application. To this end, this study was carried out to investigate the effects of inoculum concentration and steeping duration on the germinability enhancement of seeds primed in broth cultures of growth-promoting Bacillus species isolated from soil rhizospheres. The experimental crops used in this study were selected due to their economic importance in Nigeria.
2. MATERIALS AND METHODS
2.1. Bacterial Isolation
The bacterial strains used for the study were isolated from rhizospheres within Afe Babalola University, Ado-Ekiti, Nigeria. Isolation was carried out using the standard pour-plating technique. Representative colonies from the pour plating were streaked on nutrient agar plates to obtain pure cultures. The pure cultures were then stored as nutrient agar slants at 4 oC ± 2 oC until when needed.
2.2. Test Seeds
A total of 5 seeds comprising cowpea (Vigna unguiculata), soya bean (Glycine max), sorghum (Sorghum bicolor), sesame (Sesamum indicum), and okra (Abelmoschus esculentus) were used in this study and were obtained from a local market in Ado-Ekiti, Ekiti State, Nigeria and used for this study.
The seeds were identified and authenticated at the Herbarium of the Department of Plant Sciences, University of Ilorin, Kwara State, Nigeria and the following voucher numbers were obtained: cowpea (UICH/004/1491/2022), soya bean (UILH/003/1490/2022), sorghum (UILH/002/1489/2022), sesame (UILH/005/1492/2022), and okra (UILH/001/ 1488/2022).
Seeds were surface-sterilized in solution of 5% sodium hypochlorite (v/v) for 5 min and viability-tested before use. The surface-sterilized seeds were then washed three times in sterile distilled water.
For preliminary viability testing, approximately 500 seeds were soaked in a 500-mL beaker containing distilled water. Floated seeds were considered non-viable and discarded. Further viability was ascertained by planting seeds (100 seeds in triplicates) that passed the preliminary test in transparent plastic containers (80 cm in diameter and 40 cm depth) containing 3.5 g absorbent cotton wool as blotters and incubated for five days. Only seed lots with a minimum of 70% average germination (60% for okra) were considered viable and used for the study.
2.3. Germinability and Growth Promotion Studies
For the determination of optimal steeping duration, the respective seeds were steeped in 48-hour broth cultures of the respective test bacterial species and allowed to stand. Seven seeds primed in the respective inoculum were withdrawn every 1 h for a 5 h duration, sown in respective plastic cups and incubated at 25 oC ± 2 oC under fluorescent light for 8 d to assess germinability and growth parameters. In the case of inoculum concentration, the seeds were primed in different dilutions of cultures, expressed as water/broth culture ratios. The water/broth culture ratios used for the study were 4:1, 3:2, 2:3, 1:4, and 0:5. The steeping duration used in the second experiment (concentration) was the optimal steeping duration obtained in the first experiment (steeping duration), i.e., the lowest steeping duration that gave the best significant vigor index value. Following steeping, the respective seeds were planted, as described earlier. For both experiments, the following parameters were calculated and recorded.
Final germination percentage (FGP), mean germination time (MGT), germination index (GIX) and vigor index (VIX) were estimated thus:
- Final germination % (FGP) = total number of germinated seeds / total number of seeds sown x 100% [31]
-
Mean germination time (MGT) =
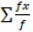 [32]
[32]
Where f is the number of seeds germinated on day x
- Germination index (GIX) = (8 × N1)+(7 × N2)+(6 × N3)+...+(1 × N8) [33]
Where N1, N2,N3...N8 represent the number of seeds that germinated on the first, second, and third until the 8th day, and 8, 9, 7…1 are the weights given to the number of germinated seeds on the first, second, and third day up to the 8th day.
- Vigor index (VIX)= FGP × average plant height [34]
2.4. Molecular Characterization of Test Bacterial Species
Extraction of DNA was carried out using 48 h old cultures grown on single colonies on medium. To obtain pellets, the resulting cultures were centrifuged at 4600 g for 5 min. Extraction and purification of the bacterial DNA were carried out by suspending the pellets in 520 μL of TE buffer (10 mMTrisHCl, 1 mM EDTA, pH 8.0). After suspension, 15 μl of 20% SDS and 3 μL of Proteinase K (20 mg/mL) were then added, and the mixture was incubated at 37 ºC for 1 h before adding 100 μL of 5 M NaCl and 80 μL of a 10% CTAB solution in 0.7 M NaCl and then vortexed. The suspension was then incubated for 10 min at 65 ºC and incubated on ice for 15 min, after which chloroform: isoamyl alcohol (24:1) mix was added before incubation on ice for 5 min and centrifugation at 7200 g for 20 min. After centrifugation, the aqueous phase was then transferred to a new tube and isopropanol (1: 0.6) was added and DNA precipitated at –20 ºC for 16 h. DNA was collected by centrifugation at 13000g for 10 min. The resulting DNA was later washed with 500 μl of 70% ethanol, air-dried at room temperature for approximately three hours, and finally dissolved in 50 μl of TE buffer.
Polymerase chain reaction (PCR) was carried out in a GeneAmp 9700 PCR System Thermal cycler (Applied Biosystem Inc., USA). Amplification of extracted DNA was carried out in a cocktail mixture that consisted of 10 µl of 5x GoTaq colourless reaction, 3 µl of 25mM MgCl2, 1 µl of 10 mM of dNTPs mix, 1 µl of 10 pmol each 27F 5’ AGA GTT TGA TCM TGG CTC AG3’ and 1525R, 5′AAGGAGGTGATCCAGCC3′ primers and 0.3units of Taq DNA polymerase (Promega, USA). The mixture was made up to 42 µl with sterile distilled water 8μl DNA template. Amplification during initial denaturation and enzyme activation took place at 94 °C for 5 min; which was followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 60 s and extension at 72°C for 90 s; and final termination at 72 °C for 10 min and chilling at 4 °C GEL.
The integrity of the amplified about 1.5Mb gene fragment was checked on a 1% Agarose gel run to confirm amplification. To separate the base pairs, 1.5% agarose gel electrophoresis in buffer (1XTAE buffer) was used. The sizes of the PCR products were estimated by comparing them to the mobility of a 100bp molecular weight ladder that was run in the gel alongside the experimental samples. Following gel integrity, the amplified fragments were purified using ethanol precipitation [35].
The amplified fragments were sequenced using an Applied Biosystems Genetic Analyzer 3130XL sequencer and the BigDye Terminator v3.1 cycle sequencing kit, according to the manufacturer's instructions. Bio Edit software and MEGA 6 were used for all genetic analyses. Sequences were deposited in the NCBI database, and ascension numbers were obtained.
2.5. Statistical Analysis
All data were presented as means and standard deviations of triplicate analysis. Comparison of means was carried out using the One-Way Analysis of Variance (ANOVA) test, with multiple comparisons carried out using the Least Significant Difference (LSD) test. All analyses were carried out at 95% confidence level.
3. RESULTS
3.1. Test Bacterial Species
Sequencing of the amplified PCR products of the bacterial isolates revealed three species of B. cereus and one of B. thuringiensis (Table 1).
| Isolate Code | Scientific Name |
Max Score |
Total Score | Query Cover | E-value | Percent Identity |
Accession Number |
|---|---|---|---|---|---|---|---|
| A | Bacillus cereus | 1615 | 1615 | 98% | 0 | 99.77% | OP830500 |
| B | Bacillus cereus | 1655 | 1655 | 100% | 0 | 100.00% | OP830493 |
| C | Bacillus cereus | 1633 | 1633 | 99% | 0 | 99.89% | OP830502 |
| D | Bacillus thuringiensis | 1626 | 1626 | 100% | 0 | 99.66% | OP830494 |
| E | Bacillus cereus | 1635 | 1635 | 99% | 0 | 99.78% | OP830495 |
3.2. Effect of Priming Duration on Germinability of the Seeds
3.2.1. Final Germination
For the cowpea seeds, final germination did not differ significantly at the different steeping durations in the presence of isolates A and B. When seeds were primed in isolates C and D, 5 h and 1, 2, and 3 h steeping durations were observed to show significantly higher percent germination than seeds steeped at other durations, respectively. For seeds that were treated in isolate E, significantly higher final percent germination was observed for seeds that were steep-primed for either 2 or 4 h (Table 1). In the case of the soybean seeds, significantly higher final germination was observed at 1 and 3 h steeping durations for isolate A. For seeds steeped in isolates B and C, significantly higher germination was observed at 1 and 4 h and 1, 2, 4, and 5 h steeping durations, respectively. However, seeds steeped in isolate E did not show a significant difference in final germination at the respective steeping durations, while 1, 2, 3, and 4 h steeping durations were observed to show significantly higher final germination when seeds were treated with isolate D (Table 2).
| - | Time | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Cowpea | 1 h | 71.43a(±15.65) | 78.57a(±7.82) | 85.70ac(±0.00) | 64.29ab(±7.82) | 73.21a(±1.96) |
| 2 h | 78.57a(±7.82) | 78.55a(±23.50) | 78.57a(±7.82) | 78.57b(±23.47) | 92.86b(±7.82) | |
| 3 h | 78.57a(±23.47) | 57.14a(±15.65) | 78.57a(±7.82) | 64.29ab(±7.82) | 64.29a(±7.82) | |
| 4 h | 71.43a(±0.00) | 71.43a(±15.65) | 64.29b(±7.82) | 57.14a(±15.65) | 78.57ab(±23.47) | |
| 5 h | 71.43a(±15.65) | 64.30a(±23.44) | 92.86c(±7.82) | 71.43ab(±0.00) | 42.86c(±15.65) | |
| Soybean | 1 h | 71.43a(±0.00) | 85.71a(±0.00) | 64.25ab(±7.83) | 64.29ac(±23.47) | 52.68a(±10.76) |
| 2 h | 50.00b(±7.82) | 64.29bc(±7.82) | 64.29ab(±7.82) | 71.43ab(±0.00) | 71.43a(±0.00) | |
| 3 h | 64.29a(±7.82) | 57.14b(±15.65) | 57.14a(±15.65) | 85.71b(±15.65) | 57.14a(±31.30) | |
| 4 h | 50.00b(±7.82) | 71.43ab(±15.65) | 71.43b(±15.65) | 85.71b(±15.65) | 71.43a(±0.00) | |
| 5 h | 50.00b(±7.82) | 50.00c(±23.44) | 64.29ab(±7.82) | 71.43bc(±15.65) | 57.14a(±15.65) | |
| Sorghum | 1 h | 92.86ac(±7.82) | 71.43a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 66.07a(±9.78) |
| 2 h | 100.00a(±0.00) | 92.86b(±7.82) | 100.00a(±0.00) | 92.86b(±7.82) | 100.00b(±0.00) | |
| 3 h | 85.71bc(±15.65) | 92.86b(±7.82) | 100.00a(±0.00) | 100.00a(±0.00) | 92.86b(±7.82) | |
| 4 h | 92.86ab(±7.82) | 100.00c(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 78.57c(±23.47) | |
| 5 h | 100.00a(±0.00) | 100.00c(±0.00) | 100.00a(±0.00) | 92.86b(±7.82) | 100.00b(±0.00) | |
| Sesame | 1 h | 92.86a(±7.82) | 92.86a(±7.82) | 92.85a(±7.83) | 64.29a(±7.82) | 86.61a(±0.98) |
| 2 h | 100.00b(±0.00) | 92.86a(±7.82) | 100.00a(±0.00) | 100.00b(±0.00) | 92.86b(±7.82) | |
| 3 h | 100.00b(±0.00) | 92.86a(±7.82) | 100.00a(±0.00) | 100.00b(±0.00) | 100.00c(±0.00) | |
| 4 h | 92.86a(±7.82) | 100.00a(±0.00) | 71.43b(±0.00) | 100.00b(±0.00) | 92.86b(±7.82) | |
| 5 h | 85.71c(±0.00) | 100.00a(±0.00) | 78.57b(±23.47) | 92.86c(±7.82) | 100.00c(±0.00) | |
| Okra | 1 h | 28.57ab(±15.65) | 7.14a(±7.82) | 7.15a(±7.83) | 50.00a(±23.47) | 20.54a(±8.80) |
| 2 h | 21.43b(±23.47) | 42.86b(±15.65) | 35.71b(±7.82) | 50.00a(±23.47) | 14.29a(±15.65) | |
| 3 h | 28.57ab(±0.00) | 21.43ab(±23.47) | 35.71b(±7.82) | 28.57ab(±15.65) | 21.43a(±7.82) | |
| 4 h | 28.57ab(±0.00) | 21.43ab(±23.47) | 28.57b(±15.65) | 21.43b(±7.82) | 42.86b(±15.65) | |
| 5 h | 42.86a(±0.00) | 42.85ab(±31.27) | 35.71b(±7.82) | 28.57ab(±15.65) | 7.14a(±7.82) |
When the sorghum seeds were treated with the isolates, significantly higher final germinations were observed at 2 and 5 h and 4 and 5 h steeping durations for isolates A and B, respectively. However, no significant difference in final germination was observed at the respective steeping durations in the presence of isolate C. In the presence of isolates D and E, 1, 3, and 4 h steeping durations and 2, 3, and 5 h steeping durations were observed to show significantly higher final germination, respectively (Table 1). Final germination of sesame seeds showed significantly higher final germination at 2 and 3 h steeping durations when treated with isolate A, while no significant difference in final germination was observed at the different steeping times when treated with isolate B. When seeds were steep-primed in isolate C, significantly higher final germination was observed at 1, 2, and 3 h steeping durations. However, when the sesame seeds were treated with isolates D and E, significantly higher germinations were observed at 2-4 h and 3 and 5 h steeping durations, respectively (Table 2).
Also, when the okra seeds were treated with the respective isolates, significantly higher final germination was observed at 1, 3, 4, and 5 h and 4 h steeping durations in the presence of isolates A and E, respectively. In the presence of isolates B and C, final germination was observed to be significantly higher when seeds were steeped for 2, 3, 4 or 5 h, while a steeping duration of 1, 2, 3, and 5 h showed significantly higher final germination in the presence of isolate D (Table 2).
3.2.2. Mean Germination Time
With respect to mean germination time, significantly lower mean germination time was observed for cowpea seeds that were steeped before planting for 1-3 h in inoculum A, 1 and 3 h in inoculum B, and 1, 2, and 4 h in inoculum C. No significant difference in mean germination time was observed at the different steeping times for seeds treated in inoculum D. For seeds primed in isolate E, significantly lower mean germination times were observed at 1, 2, 3, and 4 h steeping durations (Table 2). For the soybean seeds, significantly lower mean germination times were recorded at steeping durations of 1-3 and 5 h and 1, 2, 3, and 4 h and 1-4 h and 1-3 and 5 h and 1, 2, 4, and 5 h for seeds treated in inoculums A, B, C, D, and E, respectively (Table 3).
For the sorghum seeds, significantly lower mean germination time was observed for seeds steeped for 3 and 5 h in inoculum A. For seeds that were treated in inoculums B and D, significantly lower mean germination times were observed at 2-4 h and 2-5 h, while for seeds treated with inoculums C and E, significantly lower mean germination times were observed at 2, 3, and 5 h steeping times for both isolates (Table 3).
In the case of the sesame seeds, significantly lower mean germination times were observed at 1 and 3 h in seeds treated with inoculum A. For seed primed in inoculum B, significantly lower mean germination times were observed at the steeping duration of 2 h. In the case of inoculum C, significantly lower mean germination times were observed at steeping durations of 2 and 3 h. For inoculum D, 1, 2, and 4 h posted significantly lower mean germination times. For seeds steep-primed in inoculum E, significantly lower mean germination times were observed at 1 and 2 h. In the case of okra, significantly lower mean germination times of 2 h and 1, 2, 3, and 4 h and 1, 2, 3, and 5 h were observed for seeds steeped in isolates A, B, C, and E, respectively. In contrast, there was no significant difference between the mean germination times for the different steeping durations for seeds steeped in isolate D (Table 3).
3.2.3. Germination Index
At the different treatments, the germination index of the cowpea seeds showed significantly higher values at steeping durations of 1-3 h (inoculum A), 1-2 h (inoculum B), 1 and 5 h (inoculum C) and 1, 2, 3, and 5 h (inoculum D), and 1 and 2 h (inoculum E). For the soybean seeds, significantly higher germination index values were observed at steeping durations of 1 h (inoculum A), 1, 2, and 4 h (inoculum B), and 1, 2, 4, and 5 h (inoculum C). For seeds that were primed in inoculums D and E, there was no significant difference in the germination index of the soybean seeds at the respective steeping durations (Table 4).
| - | Time | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Cowpea | 1 h | 5.29a(±0.20) | 5.15a(±0.06) | 5.23a(±0.00) | 5.48a(±0.18) | 5.52a(±0.07) |
| 2 h | 5.29a(±0.12) | 5.60b(±0.55) | 5.35a(±0.17) | 5.53a(±0.18) | 5.53a(±0.17) | |
| 3 h | 5.31a(±0.22) | 5.32ab(±0.02) | 5.48b(±0.25) | 5.50a(±0.20) | 5.39a(±0.18) | |
| 4 h | 5.57b(±0.11) | 5.53b(±0.03) | 5.26a(±0.03) | 5.52a(±0.14) | 5.56a(±0.11) | |
| 5 h | 5.82c(±0.14) | 5.49b(±0.05) | 5.46b(±0.16) | 5.65a(±0.17) | 5.80b(±0.21) | |
| Soybean | 1 h | 5.54a(±0.05) | 5.75ab(±0.13) | 5.54ab(±0.05) | 5.63ab(±0.14) | 5.54a(±0.05) |
| 2 h | 5.61ab(±0.12) | 5.67ac(±0.23) | 5.55a(±0.10) | 5.55a(±0.04) | 5.50a(±0.00) | |
| 3 h | 5.52a(±0.07) | 5.82ad(±0.35) | 5.57a(±0.08) | 5.57a(±0.07) | 6.00b(±0.55) | |
| 4 h | 5.75bc(±0.27) | 5.61a(±0.12) | 5.57a(±0.08) | 5.79b(±0.32) | 5.50a(±0.00) | |
| 5 h | 5.61ac(±0.04) | 5.88bcd(±0.13) | 5.69b(±0.09) | 5.59a(±0.02) | 5.57a(±0.08) | |
| Sorghum | 1 h | 5.37a(±0.18) | 5.49a(±0.03) | 5.33a(±0.07) | 5.32a(±0.09) | 5.27ac(±0.21) |
| 2 h | 5.36a(±0.04) | 5.13bc(±0.07) | 5.10b(±0.04) | 5.11b(±0.12) | 5.12b(±0.01) | |
| 3 h | 5.28ab(±0.09) | 5.10b(±0.11) | 5.12b(±0.13) | 5.26ab (±0.21) | 5.08b(±0.03) | |
| 4 h | 5.40a(±0.02) | 5.13b(±0.07) | 5.33a(±0.07) | 5.20ab(±0.08) | 5.35a(±0.12) | |
| 5 h | 5.17b(±0.19) | 5.23c(±0.12) | 5.19b(±0.06) | 5.24ab(±0.10) | 5.15bc(±0.03) | |
| Sesame | 1 h | 5.27a(±0.05) | 5.37a(±0.15) | 5.33a(±0.01) | 5.35ab(±0.13) | 5.17a(±0.11) |
| 2 h | 5.37bc(±0.11) | 5.12b(±0.13) | 5.27b(±0.08) | 5.29ac(±0.03) | 5.21ab(±0.06) | |
| 3 h | 5.31ab(±0.12) | 5.25c(±0.02) | 5.27b(±0.00) | 5.38bc(±0.12) | 5.30bc(±0.11) | |
| 4 h | 5.41cd(±0.01) | 5.30ac(±0.04) | 5.43c(±0.04) | 5.27a(±0.00) | 5.37c(±0.06) | |
| 5 h | 5.50d(±0.00) | 5.38a(±0.04) | 5.50d(±0.00) | 5.54c(±0.04) | 5.50d(±0.00) | |
| Okra | 1 h | 5.41a(±0.10) | 2.75a(±3.01) | 2.50a(±2.74) | 5.34a(±0.28) | 5.70ab(±0.22) |
| 2 h | 3.00b(±3.29) | 5.10ab(±0.11) | 5.08b(±0.08) | 5.33a(±0.37) | 3.00a(±3.29) | |
| 3 h | 5.25a(±0.27) | 2.63a(±2.88) | 5.40b(±0.44) | 5.22a(±0.24) | 5.25ab(±0.27) | |
| 4 h | 5.00a(±0.00) | 3.25ac(±3.56) | 6.26b(±0.81) | 5.55a(±0.05) | 6.02b(±0.57) | |
| 5 h | 5.64a(±0.31) | 5.94bc(±0.61) | 5.08b(±0.09) | 5.50a(±0.55) | 3.50ab(±3.83) |
| - | Time | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Cowpea | 1 h | 116.00ac(±12.05) | 140.50ac(±8.22) | 147.00a(±0.00) | 93.00ab(±1.10) | 111.50ab(±13.69) |
| 2 h | 129.50a(±3.83) | 114.50ab(±73.94) | 118.50b(±0.55) | 110.00b(±20.81) | 130.00a(±4.38) | |
| 3 h | 124.00a(±20.81) | 88.50b(±24.65) | 104.50b(±16.98) | 92.50ab(±0.55) | 99.00b(±1.10) | |
| 4 h | 98.00bc(±8.76) | 97.00bc(±14.24) | 108.50b(±11.50) | 84.50a(±30.12) | 106.00b(±23.00) | |
| 5 h | 83.50b(±24.65) | 91.00b(±29.58) | 137.00a(±27.39) | 94.00ab(±12.05) | 52.50c(±27.93) | |
| Soybean | 1 h | 102.00a(±3.29) | 102.50ac(±9.31) | 91.50ab(±s8.22) | 89.00a(±40.53) | 81.00a(±19.72) |
| 2 h | 66.00b(±3.29) | 83.00ab(±2.19) | 89.50ab(±4.93) | 100.00a(±1.10) | 105.00a(±0.00) | |
| 3 h | 92.00c(±7.67) | 75.50bd(±16.98) | 81.00a(±26.29) | 120.00a(±16.43) | 73.00a(±58.06) | |
| 4 h | 60.00b(±3.29) | 96.00a(±13.15) | 99.00b(±16.43) | 108.50a(±42.17) | 105.00a(±0.00) | |
| 5 h | 68.00b(±12.05) | 57.00cd(±29.58) | 82.50ab(±4.93) | 99.00a(±23.00) | 81.00a(±26.29) | |
| Sorghum | 1 h | 143.50a(±4.93) | 103.50a(±2.74) | 161.50a(±7.12) | 160.00ac(±9.86) | 123.00a(±41.63) |
| 2 h | 158.50ab(±3.83) | 168.00bd(±7.67) | 185.50b(±3.83) | 169.00ab(±1.10) | 182.50b(±0.55) | |
| 3 h | 144.00a(±33.96) | 171.50be(±3.83) | 182.50b(±14.79) | 169.00b(±21.91) | 172.50b(±11.50) | |
| 4 h | 144.00a(±10.95) | 182.00c(±7.67) | 161.50a(±7.12) | 175.00bc(±7.67) | 127.00a(±46.01) | |
| 5 h | 178.50b(±19.17) | 171.50de(±11.50) | 175.50b(±7.12) | 156.00bd(±2.19) | 179.00b(±3.29) | |
| Sesame | 1 h | 154.50ab(±8.22) | 147.00ac(±0.00) | 150.50a(±11.50) | 102.00a(±4.38) | 164.50a(±3.83) |
| 2 h | 158.00a(±10.95) | 171.50b(±26.84) | 168.00a(±7.67) | 165.00b(±3.29) | 161.00a(±7.67) | |
| 3 h | 164.50a(±11.50) | 157.50ab(±11.50) | 168.00a(±0.00) | 158.00b(±10.95) | 165.00a(±10.95) | |
| 4 h | 143.50b(±11.50) | 164.50b(±3.83) | 109.00b(±3.29) | 168.00b(±0.00) | 147.00b(±7.67) | |
| 5 h | 126.00c(±0.00) | 157.50bc(±3.83) | 115.50b(±34.51) | 133.50c(±14.79) | 147.00b(±0.00) | |
| Okra | 1 h | 45.50a(±26.84) | 10.50a(±11.50) | 14.00a(±15.34) | 83.50a(±54.22) | 26.00a(±5.48) |
| 2 h | 22.50b(±24.65) | 77.50b(±23.55) | 66.50b(±11.50) | 73.00ac(±18.62) | 13.50b(±14.79) | |
| 3 h | 49.00a(±7.67) | 35.50ac(±38.89) | 52.00bc(±4.38) | 46.00bc(±19.72) | 35.00ac(±7.67) | |
| 4 h | 56.00a(±0.00) | 15.00a(±16.43) | 32.50ac(±29.03) | 27.50b(±7.12) | 39.50c(±2.74) | |
| 5 h | 51.00a(±12.05) | 57.50bc(±52.03) | 65.00b(±20.81) | 35.00b(±7.67) | 3.00d(±3.29) |
| - | Time | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Cowpea | 1 h | 1065.71a(±254.41 | 1146.73a(±91.88) | 1209.80ac(±210.59 | 649.29a(±163.98 | 883.34a(±190.78) |
| 2 h | 1286.22a(±328.07 | 1121.02a(±897.15 | 1079.90ab(±5.25) | 676.94a(±382.06 | 1251.63b(±134.36) | |
| 3 h | 1093.47a(±523.58 | 357.96b(±69.53) | 1028.88ab(±49.97) | 705.00a(±194.61 | 478.67cd(±60.25) | |
| 4 h | 658.67b(±38.56) | 775.31ab(±139.28 | 823.06b(±164.76 | 664.90a(±341.38 | 715.10ac(±330.87) | |
| 5 h | 592.45b(±162.53 | 713.57ab(±518.10 | 1526.63c(±585.84 | 818.37a(±421.41 | 249.59d(±233.62) | |
| Soybean | 1 h | 807.14a(±247.03 | 686.94a(±130.11 | 847.35a(±135.92 | 755.82ab(±498.65) | 419.32ac(±191.07) |
| 2 h | 295.41b(±119.05 | 249.18b(±104.85 | 338.16b(±29.73) | 672.96b(±67.63) | 890.31bd(±82.16) | |
| 3 h | 420.10b(±256.31 | 288.47b(±85.29) | 396.94b(±260.45 | 1175.82a(±56.23) | 662.45abc(±641.17) | |
| 4 h | 270.31b(±114.35 | 527.96a(±90.99) | 414.90b(±88.75) | 1132.65a(±605.85 | 793.88ab(±263.80) | |
| 5 h | 285.82b(±94.01) | 326.22b(±238.43 | 476.53b(±90.54) | 742.45c(±160.52 | 356.63c(±159.29) | |
| Sorghum | 1 h | 932.45a(±255.53 | 437.24a(±77.69) | 1252.14a(±197.96 | 1223.57a(±35.21) | 501.38a(±349.82) |
| 2 h | 910.00a(±17.21) | 1013.06b(±370.66 | 1092.14b(±21.13) | 1039.18b(±235.63 | 1215.00b(±75.90) | |
| 3 h | 738.17a(±211.71 | 732.86c(±164.32 | 1180.71a(±127.54 | 921.43bc(±29.73) | 897.76cd(±127.65) | |
| 4 h | 899.08a(±259.22 | 787.86bc(±138.50 | 1027.14c(±39.12) | 916.43bc(±7.04) | 642.55ac(±396.26) | |
| 5 h | 932.14a(±258.99 | 852.14bc(±66.51) | 905.71d(±23.47) | 798.37c(±195.84 | 1162.14bd(±82.16) | |
| Sesame | 1 h | 461.94a(±33.87) | 467.96a(±44.49) | 544.80a(±40.13) | 243.06a(±17.66) | 478.34ac(±20.99) |
| 2 h | 469.29a(±2.35) | 513.78a(±171.14 | 499.29a(±11.74) | 520.00b(±14.08) | 634.90b(±112.00) | |
| 3 h | 443.57a(±58.68) | 448.78a(±6.04) | 519.29a(±2.35) | 511.43b(±7.82) | 576.43ab(±41.47) | |
| 4 h | 379.69b(±83.28) | 519.29a(±25.82) | 256.12b(±10.06) | 529.29b(±27.39) | 556.12ab(±126.31) | |
| 5 h | 297.55c(±37.56) | 322.14b(±7.04) | 282.45b(±166.33 | 354.08c(±0.22) | 450.00c(±78.25) | |
| Okra | 1 h | 67.04ab(±43.26) | 10.51ad(±11.51) | 14.90a(±16.32) | 356.73a(±331.32 | 37.19ab(±16.49) |
| 2 h | 53.88a(±59.02) | 185.92b(±146.88 | 160.10b(±72.10) | 228.98ab(±135.92 | 17.76ad(±19.45) | |
| 3 h | 79.80ab(±6.93) | 72.86abc(±79.81) | 158.37b(±66.62) | 96.22b(±56.90) | 56.84b(±42.81) | |
| 4 h | 64.08ab(±5.37) | 43.47ac(±47.62) | 104.69b(±110.66 | 37.24b(±10.62) | 125.51c(±45.83) | |
| 5 h | 96.12b(±14.08) | 164.90bc(±170.35 | 91.12ab(±44.38) | 102.45b(±86.96) | 0.51d(±0.56) |
In the case of the sorghum seeds, significantly higher germination index values were observed at 2 and 5 h and 4 h in seeds steeped in inoculums A and B, respectively, while for those steeped in inoculums C and E, significantly higher germination index values were observed at steeping durations of 2 and 3 h and 2, 3 and 5 h, respectively. For inoculum D steeped seeds, 1 and 4 h steeping durations showed significantly higher germination index. Furthermore, the germination index of the sesame seeds showed significantly higher values at steeping times of 1-3 h (inoculum A), 2-5 h (inoculum B), 1-3 h (inoculum C), 2-4 h (inoculum D), and 1-3 h (inoculum E). For the okra seeds, significantly lower germination index values were observed at 2 and 5 h in the presence of inoculums A and E, respectively. However, significantly higher germination index values were observed at 2 and 5 h (inoculum B), 2, 3, and 5 h (inoculum C), and 1 and 2 h (inoculum D) (Table 4).
3.2.4. Vigor Index
As shown in Table 4, significantly higher vigor index values were observed in cowpea seeds that were steeped between 1-3 h and 1, 2, 4, and 5 h and 1 and 5 h and 2 h in inoculums A, B, C and E, respectively. No significant difference in vigor index values was observed for seeds steeped in inoculum D. For the soybean seeds, 1 h, 1 and 4 h, and 1 h durations showed significantly higher vigor index when treated in inoculums A, B and C, respectively. In the case of soybean seeds steeped in inoculums D and E, a significantly higher vigor index was observed at steeping periods of 1, 3, and 4 h for both isolates. In the case of the sorghum seeds, no significant difference in vigor index was observed for seeds steeped in inoculum A at the respective durations. For seeds treated with inoculums B, C, D and E, 2, 4, and 5 h, 2 h, 2, 3, and 4 h, and 2 and 4 h steeping durations were observed to have significantly higher vigor index values, respectively. Vigor index of the sesame seeds showed a significantly higher vigor index at steeping durations of 1-3 h, 1-4 h, 1-3 h, 2-4 h, and 2-4 h when treated with inoculums A, B, C, D and E, respectively. With respect to the okra seeds, significantly higher vigor index values were observed at steeping durations of 1, 3-5 h, 2, 3 and 5 h, 2-5 h, 1 and 2 h, and 4 h for seeds treated in inoculums A, B, C, D and E, respectively (Table 5).
3.3. Effect of Initial Inoculum Concentration on Final Germination of the Seeds
For the isolate-treated cowpea seeds, final germination was not observed to be concentration-dependent. However, significantly higher final germinations were observed at initial inoculum concentrations of 3:2 and 4:1, 3:2, 2:3, and 1:4 and 4:1 and 3:2, 1:4, and 0:5 and 4:1, 3:2, 2:3, and 1:4 when inoculums A, B, C, D, and E were used for priming, respectively (Table 6). In the case of the soybean seeds, no significant difference in final germination was observed in the presence of inoculum C at the different concentrations. However, significantly higher final germination was observed when 3:2, 2:3, 1:4, and 0:5 dilutions of inoculum A were used. The 0:5 dilution of inoculum D gave significantly higher final germination. For inoculums E and B, 2:3 dilutions and 3:2, 2:3, and 1:4 dilutions produced significantly higher final germination (Table 6).
Also, the final germination of the sorghum and sesame seeds was observed to be remarkable at the respective inoculum concentrations used. This observation was irrespective of the bacterial isolate. Generally, germination of over 80% was observed for seeds at the respective treatments. For the okra seeds, significantly higher final germination was observed at inoculum concentrations of 3:2 and 0:5 dilutions, 4:1, 2:3, and 1:4 dilutions, 1:4 dilution, 3:2, 2:3, 1:4, and 0:5 dilutions, 4:1, 2:3, and 0:5 dilutions, when A, B, C, D, and E, respectively, were used for treatment (Table 6).
| - | - | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Cowpea | C1 | 50.00ac(±7.78) | 85.71a(±15.65) | 92.86a(±7.82) | 64.29a(±7.82) | 57.14a(±15.65) |
| C2 | 64.29b(±7.82) | 78.57ab(±7.82) | 64.29b(±7.82) | 71.43ab(±15.65) | 57.14a(±15.65) | |
| C3 | 57.14a(±15.65) | 92.86a(±7.82) | 64.29b(±7.82) | 57.14a(±15.65) | 57.14a(±15.65) | |
| C4 | 42.86c(±15.65) | 85.71a(±15.65) | 64.29b(±39.12) | 85.71b(±0.00) | 50.00a(±7.82) | |
| C5 | 50.00a(±7.82) | 64.29b(±23.47) | 57.14b(±15.65) | 71.43ab(±31.30) | 14.29b(±15.65) | |
| Soybean | C1 | 21.45a(±7.83) | 64.29a(±7.82) | 57.14a(±31.30) | 78.57a(±7.82) | 57.14a(±0.00) |
| C2 | 57.14b(±15.65) | 85.71b(±0.00) | 57.14a(±15.65) | 50.00b(±7.82) | 71.43b(±0.00) | |
| C3 | 57.14b(±31.30) | 85.71b(±0.00) | 50.00a(±7.82) | 78.57a(±7.82) | 92.86c(±7.82) | |
| C4 | 42.86b(±0.00) | 92.86b(±7.82) | 50.00a(±23.47) | 78.57a±7.82) | 57.14a(±0.00) | |
| C5 | 57.14b(±15.65) | 42.86c(±15.65) | 57.14a(±0.00) | 92.86c(±7.82) | 64.29d(±7.82) | |
| Sorghum | C1 | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 92.86a(±7.82) |
| C2 | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00b(±0.00) | |
| C3 | 100.00a(±0.00) | 92.86b(±7.82) | 85.71b±15.65) | 92.86b(±7.82) | 92.86a(±7.82) | |
| C4 | 100.00a(±0.00) | 92.86b(±7.82) | 92.86ab(±7.82) | 100.00a(±0.00) | 100.00b(±0.00) | |
| C5 | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00b(±0.00) | |
| Sesame | C1 | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) |
| C2 | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | |
| C3 | 100.00a(±0.00) | 92.86b(±7.82) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | |
| C4 | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | 100.00a(±0.00) | |
| C5 | 100.00a(±0.00) | 100.00a(±0.00) | 92.86b(±7.82) | 100.00a(±0.00) | 100.00a(±0.00) | |
| Okra | C1 | 28.60a(±0.00) | 85.71a(±15.65) | 50.00a(±7.82) | 57.14a(±15.65) | 78.57a(±7.82) |
| C2 | 92.86b(±7.82) | 64.29bc(±7.82) | 57.14a(±0.00) | 85.71b(±0.00) | 7.14b(±7.82) | |
| C3 | 78.57c(±23.47) | 78.57ab(±7.82) | 35.71b(±23.47) | 85.71b(±15.65) | 64.29ac(±7.82) | |
| C4 | 64.29d(±7.82) | 78.57ab(±7.82) | 92.86c(±7.82) | 85.71b(±0.00) | 57.14c(±15.65) | |
| C5 | 85.71b(±0.00) | 57.14c(±31.30) | 57.14a(±0.00) | 85.71b(±0.00) | 71.43ac(±31.30) |
| - | - | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Cowpea | C1 | 5.39a(±0.08) | 5.41a(±0.00) | 5.21a(±0.02) | 5.27a(±0.04) | 5.31a(±0.17) |
| C2 | 5.36a(±0.19) | 5.19b(±0.01) | 5.37b(±0.01) | 5.21b(±0.02) | 5.20a(±0.05) | |
| C3 | 5.30a(±0.02) | 5.25bc(±0.08) | 5.37b(±0.01) | 5.50c(±0.03) | 5.12a(±0.03) | |
| C4 | 5.34a(±0.12) | 5.32ac(±0.15) | 5.38b(±0.04) | 5.26a(±0.03) | 5.35a(±0.03) | |
| C5 | 5.34a(±0.02) | 5.35a(±0.03) | 5.47b(±0.24) | 5.21b(±0.06) | 2.50b(±2.74) | |
| Soybean | C1 | 5.36a(±0.40) | 5.37ab(±0.01) | 5.30a(±0.08) | 5.26a(±0.03) | 5.34a(±0.12) |
| C2 | 5.56ab(±0.27) | 5.32b(±0.00) | 5.40a(±0.05) | 5.32b(±0.06) | 5.33a(±0.06) | |
| C3 | 5.41ac(±0.20) | 5.20c(±0.14) | 5.37a(±0.12) | 5.12c(±0.03) | 5.18b(±0.06) | |
| C4 | 5.32a(±0.00) | 5.42a(±0.09) | 5.23a(±0.26) | 5.21a(±0.03) | 5.36a(±0.00) | |
| C5 | 5.66bc(±0.02) | 5.43a(±0.08) | 5.73b(±0.14) | 5.37b(±0.06) | 5.41a(±0.06) | |
| Sorghum | C1 | 5.03a(±0.03) | 5.10a(±0.04) | 5.13a(±0.00) | 5.10ab(±0.04) | 5.00a(±0.00) |
| C2 | 5.08a(±0.03) | 5.06ab(±0.07) | 5.06b(±0.00) | 5.03a(±0.03) | 5.12b(±0.06) | |
| C3 | 5.16b(±0.04) | 5.03b(±0.03) | 5.03b(±0.03) | 5.07ab(±0.01) | 5.06c(±0.07) | |
| C4 | 5.10bc(±0.11) | 5.07ab(±0.01) | 5.03b(±0.03) | 5.14b(±0.15) | 5.06c(±0.00) | |
| C5 | 5.06ac(±0.00) | 5.16c(±0.04) | 5.10c(±0.04) | 5.10ab(±0.04) | 5.06c(±0.00) | |
| Sesame | C1 | 5.27a(±0.08) | 5.27a(±0.00) | 5.34a(±0.00) | 5.36a(±0.25) | 5.27a(±0.08) |
| C2 | 5.27a(±0.00) | 5.27a(±0.08) | 5.27b(±0.08) | 5.27a(±0.08) | 5.30a(±0.04) | |
| C3 | 5.27a(±0.08) | 5.38b(±0.04) | 5.34a(±0.08) | 5.30a(±0.04) | 4.99b(±0.16) | |
| C4 | 5.20a(±0.08) | 5.37b(±0.03) | 5.27b(±0.00) | 5.30a(±0.04) | 5.27a(±0.08) | |
| C5 | 5.31a(±0.13) | 5.30a(±0.04) | 5.29ab(±0.03) | 5.30a(±0.04) | 5.23a(±0.12) | |
| Okra | C1 | 5.62a(±0.42) | 5.08a(±0.08) | 5.24ab(±0.09) | 5.44a(±0.01) | 5.19a(±0.11) |
| C2 | 5.33bc(±0.03) | 5.34b(±0.02) | 5.33a(±0.15) | 5.35b(±0.00) | 3.00b(±3.29) | |
| C3 | 5.50ab(±0.02) | 5.32b(±0.07) | 5.16b(±0.18) | 5.16c(±0.01) | 5.47a(±0.31) | |
| C4 | 5.38abd(±0.25) | 5.38b(±0.07) | 5.23ab(±0.13) | 5.36b(±0.01) | 5.31a(±0.05) | |
| C5 | 5.18cd(±0.19) | 5.49c(±0.02) | 5.34a(±0.12) | 5.17c(±0.04) | 5.36a(±0.23) |
Generally, the mean germination time of the seeds at the different concentrations of inoculums did not follow any visible trend. However, in the presence of inoculum A, no significant difference in the mean germination time of cowpea and sesame seeds was observed at the different initial inoculum concentrations. For soybean seeds, significantly lower mean germination times were observed at 2:3 dilution for inoculums B, D, and E. In the presence of inoculums A and D, no significant difference in the mean germination time of sesame seeds was observed at the different inoculums (Table 7).
The germination index of the seeds in the presence of inoculum A showed significantly higher values at inoculum dilutions of 3:2, 2:3, and 0:5 dilutions and 3:2, 2:3, 1:4, and 0:5 dilutions, for cowpea and sorghum seeds, respectively. Also, in the presence of inoculum C, significantly higher germination index values were observed at dilutions of 4:1 for cowpea seeds and 1:4 for okra seeds; however, there was no significant difference in germination index across the respective dilutions for soybean seeds. Seeds treated in inoculum D showed significantly higher germination index at dilutions of 3:2, 1:4, and 0:5 and 4:1, 3:2, 1:4, and 0:5 for cowpea and sorghum seeds, respectively, and significantly higher values were recorded at dilutions of 2:3, 1:4, and 0:5 and 3:2, 2:3, 1:4, and 0:5 for soybean and okra seeds, respectively. In addition, a significantly lower germination index was recorded for cowpea and okra seeds at dilutions of 0:5 and 3:2 for inoculum E. For seeds treated in inoculum B, a significantly higher germination index showed significantly higher values for cowpea and sesame seeds at dilutions of 3:2 and 2:3 for cowpea, 4:1, 3:2, and 0:5 for sesame seeds, and 4:1 for okra seeds (Table 8).
| - | - | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Cowpea | C1 | 77.50ac(±8.22) | 125.50ac(±23.55) | 161.00a(±15.34) | 104.50ab(±7.12) | 91.50a(±15.88) |
| C2 | 98.00b(±1.10) | 137.00ab(±12.05) | 101.50b(±11.50) | 123.00ac(±26.29) | 98.50a(±23.55) | |
| C3 | 94.50ab(±26.84) | 158.00b(±19.72) | 101.50b(±11.50) | 82.50b(±25.74) | 105.00a(±30.67) | |
| C4 | 67.00c(±19.72) | 132.00a(±6.57) | 100.00b(±62.44) | 144.00c(±3.29) | 78.50a(±9.31) | |
| C5 | 80.50abc(±11.50) | 102.00c(±35.05) | 86.50b(±36.70) | 122.50ac(±49.84) | 28.00b(±30.67) | |
| Soybean | C1 | 32.00a(±4.38) | 101.50a(±11.50) | 91.50a(±46.56) | 133.00a(±15.34) | 91.50a(±7.12) |
| C2 | 76.50b(±7.12) | 140.00b(±0.00) | 88.50a(±26.84) | 79.00b(±8.76) | 115.50b(±3.83) | |
| C3 | 82.50b(±36.70) | 150.50b(±11.50) | 76.00a(±5.48) | 143.50ac(±11.50) | 164.50c(±19.17) | |
| C4 | 70.00b(±0.00) | 143.50b(±19.17) | 81.00a(±27.39) | 136.50ac(±11.50) | 91.00a(±0.00) | |
| C5 | 75.00b(±19.72) | 66.50c(±26.84) | 72.00a(±6.57) | 147.00c(±7.67) | 98.50a(±8.22) | |
| Sorghum | C1 | 192.50a(±3.83) | 185.50ab(±3.83) | 180.00ab(±2.19) | 185.50ab(±3.83) | 182.00ab(±15.34) |
| C2 | 186.00ac(±3.29) | 189.00a(±7.67) | 189.00a(±0.00) | 192.50a(±3.83) | 182.50ab(±7.12) | |
| C3 | 178.50b(±3.83) | 178.50ab(±11.50) | 164.50bc(±26.84) | 175.00b(±15.34) | 175.00a(±7.67) | |
| C4 | 185.50cd(±11.50) | 175.00b(±15.34) | 178.50ac(±11.50) | 180.00a(±17.53) | 189.00b(±0.00) | |
| C5 | 189.00ad(±0.00) | 176.50b(±6.02) | 185.50a(±3.83) | 183.50ab(±6.02) | 189.00b(±0.00) | |
| Sesame | C1 | 168.00ab(±7.67) | 168.00a(±0.00) | 161.00ab(±0.00) | 157.50a(±26.84) | 168.00a(±7.67) |
| C2 | 168.00ab(±0.00) | 168.00a(±7.67) | 168.00a(±7.67) | 168.00a(±7.67) | 164.50a(±3.83) | |
| C3 | 168.00ab(±7.67) | 147.00b(±15.34) | 161.00ab(±7.67) | 164.50a(±3.83) | 197.00b(±16.43) | |
| C4 | 175.00a(±7.67) | 156.00bc(±1.10) | 168.00a(±0.00) | 164.50a(±3.83) | 168.00a(±7.67) | |
| C5 | 162.50b(±13.69) | 164.50ac(±3.83) | 154.00b(±15.34) | 164.50a(±3.83) | 171.50a(±11.50) | |
| Okra | C1 | 39.50a(±10.41) | 161.50a(±37.79) | 84.50a(±8.22) | 85.50a(±23.55) | 137.00ac(±4.38) |
| C2 | 149.00b(±15.34) | 102.50bc(±11.50) | 92.00a(±7.67) | 135.00b(±0.00) | 7.50b(±8.22) | |
| C3 | 112.00c(±33.96) | 127.00b(±7.67) | 60.00b(±35.05) | 151.50b(±26.84) | 181.00c(±89.83) | |
| C4 | 102.50c(±26.84) | 121.00b(±7.67) | 159.00c(±26.29) | 134.50b(±0.55) | 92.00a(±23.00) | |
| C5 | 151.50b(±18.07) | 84.50c(±46.56) | 91.50a(±7.12) | 151.50b(±3.83) | 107.00a(±32.86) |
| - | - | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Cowpea | C1 | 245.71a(±172.59) | 756.84ab(±283.59) | 979.08a(±46.39) | 435.10a(±153.81) | 296.63a(±174.71) |
| C2 | 494.59b(±6.37) | 900.41ac(±400.62) | 588.57ab(±119.83) | 705.31a(±568.74) | 285.92a(±156.27) | |
| C3 | 395.41ab(±288.95) | 1106.84a(±81.71) | 595.82ab(±5.48) | 352.65a(±297.78) | 448.06a(±167.56) | |
| C4 | 248.37a(±158.06) | 1091.33a(±314.66) | 761.53ab(±757.31) | 675.92a(±91.21) | 331.02a(±198.97) | |
| C5 | 368.27ab(±47.28) | 569.08bc(±430.91) | 396.84b(±291.86) | 730.51a(±600.37) | 49.18b(±53.88) | |
| Soybean | C1 | 19.90a(±18.44) | 720.82a(±149.34) | 397.96a(±289.73) | 962.86a(±133.69) | 413.88a(±4.47) |
| C2 | 406.43b(±253.41) | 1149.80b(±76.46) | 360.71a(±220.77) | 317.65b(±10.62) | 924.49b(±21.24) | |
| C3 | 292.45b(±212.16) | 1275.31bc(±114.7) | 350.61a(±32.64) | 1107.76a(±196.29) | 1460.61c(±453.16) | |
| C4 | 180.92ab(±90.21) | 1458.57c(±339.59) | 333.06a(±216.41) | 1025.61a(±105.19) | 617.14ad(±17.88) | |
| C5 | 379.69b(±263.69) | 152.24d(±123.85) | 297.55a(±73.77) | 1604.39c(±228.37) | 720.82bd(±149.34) | |
| Sorghum | C1 | 881.43a(±98.59) | 973.57a(±75.90) | 643.57a(±97.81) | 884.29a(±92.33) | 765.31a(±147.55) |
| C2 | 829.29a(±110.33) | 887.86a(±13.30) | 921.43b(±219.09) | 774.29a(±104.85) | 930.71b(±47.73) | |
| C3 | 615.71b(±156.49) | 992.55a(±106.75) | 754.49ab(±420.74) | 648.37b(±87.86) | 890.51b(±43.26) | |
| C4 | 837.14a(±40.69) | 925.20a(±114.80) | 714.69ab(±93.45) | 776.43a(±125.98) | 766.43a(±14.87) | |
| C5 | 1030.71c(±24.26) | 966.43a(±269.95) | 927.14b(±133.02) | 849.29a(±107.20) | 763.57a(±55.55) | |
| Sesame | C1 | 484.29a(±37.56) | 682.86a(±15.65) | 573.57a(±55.55) | 590.71a(±24.26) | 585.00a(±33.65) |
| C2 | 522.86a(±34.43) | 589.29b(±79.03) | 620.00ab(±7.82) | 668.57a(±129.89) | 575.71a(±62.60) | |
| C3 | 527.14a(±9.39) | 539.59b(±41.14) | 686.43b(±135.37) | 617.14a(±20.34) | 563.57a(±3.91) | |
| C4 | 700.00b(±75.12) | 661.43ac(±23.47) | 680.00b(±68.86) | 657.86a(±2.35) | 509.29b(±39.91) | |
| C5 | 544.29a(±84.51) | 613.57c(±22.69) | 569.59a(±27.05) | 632.14a(±68.07) | 548.57ab(±50.08) | |
| Okra | C1 | 54.49a(±9.17) | 811.53a(±290.96) | 162.35a(±5.48) | 306.53a(±139.28) | 594.90a(±79.36) |
| C2 | 704.59b(±62.26) | 437.24b(±174.94) | 284.90a(±86.74) | 596.94b(±32.86) | 102.20b(±0.22) | |
| C3 | 468.57c(±118.93) | 547.86ab(±122.85) | 163.47a(±158.06) | 684.69b(±220.21) | 428.98ac(±57.68) | |
| C4 | 346.33d(±154.93) | 511.94b(±132.68) | 875.10b(±349.65) | 435.31a(±79.81) | 281.22c(±169.24) | |
| C5 | 683.88b(±26.16) | 370.20b(±350.99) | 254.29a(±48.74) | 584.08b(±53.65) | 375.10c(±318.35) |
With respect to the vigor index of the seeds, no consistent pattern of increase or decrease was observed with a concentration of inoculum. In the presence of inoculum A, significantly higher vigor index values were observed at inoculum dilutions of 3:2, 2:3, and 0:5, 4:1, 3:2, and 1:4, and 1:4, for cowpea, sorghum and sesame seeds, respectively, while significantly lower values were recorded at dilution of 4:1 for soybean and okra seeds (Table 9).
For inoculum C-treated seeds, vigor index showed significantly lower values at inoculum concentrations of 0:5 and 4:1 for cowpea and sorghum seeds, respectively, while significantly higher values were recorded at dilution of 3:2, 2:3, and 1:4 and 1:4 for sesame and okra seeds, respectively. In seeds treated in inoculum D, vigor index values of cowpea and sesame seeds showed no significant difference across the respective dilutions, while significantly higher values were recorded at dilutions of 0:5 and 4:1, 3:2, 1:4, and 0:5, for soybean and sorghum seeds, respectively. For the inoculums B-treated seeds, no significant difference in vigor was observed for sorghum seeds at the respective concentrations. However, for cowpea, soybean, sesame, and okra seeds treated with inoculum B, significantly higher vigor index values were obtained at dilutions of 4:1, 3:2, 2:3, and 1:4, 2:3 and 1:4, 4:1 and 1:4, and 4:1 and 2:3, respectively. For soybean, sorghum, sesame seeds, and okra seeds, significantly higher vigor index values were observed when treated in dilutions of 2:3 and 3:2 and 2:3 and 4:1, 3:2, 2:3, 0:5 and 4:1 and 2:3, for inoculum E, respectively, while significantly lower values were observed for cowpea in dilution of 0:5 (Table 9).
4. DISCUSSION
The bacterial species used in this study were previously identified as plant growth promoters. The essence of this study was to determine the impact of varying steeping duration and concentration on their growth promotion activity vis-à-vis some agronomic parameters. Final germination represents the number of seeds that germinated, expressed as a percentage of seeds planted. A higher FGP signifies that a large number of seeds germinated [36]. Proper seed germination is a fundamental process in a life cycle of a plant, and it is directly linked to plant productivity [37, 38]. In this study, there was no consistent relationship between steeping duration and final germination across all the crops for the isolates. Most of the time, a higher value at a shorter steeping duration was also found to be statistically similar to values obtained at longer steeping durations. Nevertheless, in this study, for cowpea and soybean, longer steeping durations tended to post lower final percent germination due to over-imbibition, which resulted in sogginess of the seeds, and hence, impeded germination. Therefore, while longer steeping durations can provide more time for further bacterial attachment to the seed surface, which may prove beneficial down the line after seeds are sown, there is the risk of over-imbibition when seeds (such as cowpea and soybean) with large hydrophilic endosperms are sown directly without drying. Again, a high bacterial population on seed, which is possible with a longer steeping period, can reduce seed energy efficiency since more bacteria means more energy is needed for their proliferation [39]. Moreover, longer steeping periods can soften these seeds to the point where bacterial and fungal deterioration becomes likely after sowing.
According to Okamoto and Joly [40], a prolonged priming duration can even lead to hypoxia which can reduce percent germination. Nonetheless, some studies have identified the growth promotion potential of some bacterial species in the area of germination percentage. In a study by Pooja et al. [41], chicken pea seeds (a member of the legume family) bio-primed with Bacillus valezensis (MNB08) were found to show enhanced final germination. The application of Bacillus velezensis strain CMRP 4490 as a coating film on soybean seeds also improved germination [42].
Using the conventional priming method, which involves steeping and then drying seeds to their original moisture content, cowpea seeds bio-primed for 6 hours with either Trichoderma viridae or Pseudomonas florescence gave improved germination percentage [43]. In another study by Aminu et al. [44], where 3 different priming durations (4, 6, and 8 h) were used, 8 h was found to be the priming duration for superior performance in terms of stand count and plant heights after 8 weeks of sowing for two varieties of soybean at two different locations. The results of hydropriming experiments by Mehri [45] revealed that 18 hours of priming was necessary for improved final germination and yield in soybean.
The high optimal priming durations reported in these studies are not unusual, even if they are not consistent with the results in this study, because these studies used the conventional priming technique. Seeds in the current study were planted directly, hence the lower optimal steeping duration was reported. The lack of a consistent relationship between steeping duration and final germination for sorghum and sesame, two important crops well-adapted for growth under arid conditions, could be linked to their seed structures which perhaps limited over-imbibition, which could have occurred at longer steeping durations, as FPG of 100% (or close to) were obtained for virtually all the isolates, even at longer steeping durations (i.e., 4 and 5 hours). That is, longer steeping durations did not result in bloated seeds which were visually observed for cowpea and soybean in this study. Ituen et al. [46] discovered that the sorghum took 48 h to reach full water absorption capacity, showing the imperviousness of the seed. The best hydropriming duration for improved germination performance in sesame was 12 hours [47]. Teshome [48] reported an increase in final percent germination as priming duration increased for virtually all varieties of the sorghum tested against all priming treatments, up to 10 hours, then it decreased. Tizazu et al. [49] also recorded the best mean germination value (91.5%) for seeds primed in water for 12 hours for sesame using the convention priming method. Since the main goal of bacterial seed priming is the adherence of beneficial species to the spermosphere and imbibition of water and, to a much lesser extent, bioactive bacterial metabolites that can promote plant growth; therefore, longer steeping durations were not utilized in this study.
Generally, rather low FPG values were obtained in this study for okra. It ranged from a measly 7.14 to 64.29% for the isolates. Lethargic and irregular germination pattern has been a major problem in okra production due to the hardness of the seed coat [50-52]. This slows down the imbibition of liquid, meaning that seemingly viable seeds could have slow, erratic, and uneven germination patterns. The hydropriming basil seeds, a very hardy seed like okra, required 12 hours of priming to produce a significant effect [53] but in this study, the highest steeping duration was 5 hours. In the case of okra, it is possible that longer steeping periods would have given rise to a more improved germination pattern. Softer seeds will even promote bacterial adherence since a longer steeping durations will soften the seed.
Mean germination time indicates the germination rate and time spread of germination [54]. It gives information about the average time taken for germinated seeds to appear. The lower the MGT, the quicker the germination of a seed lot. Two seed lots can have different FGPs but the same MGT and vice versa. This implies that one parameter must be interpreted in light of the other. In this study, a stable value of around 5 days, with very few exceptions across the different steeping durations for the 5 crops. As an example, rather low values were obtained at 2 h (inoculum A), 1, 3, and 4 h (inoculum B), 1 h (inoculum C), and 2 and 5 h (inoculum E). These low values should be interpreted in the light of FPG, which was low for all of them. This implies that low FPG can generate misleadingly low mean germination time.
In this study, no observable patterns with steeping duration were obtained for the isolates across all crops. In other words, the impact of increasing steeping duration on mean germination time was minimal. However, some researchers have found reduced mean germination time in their studies through different priming methods [55-57].
The germination index combines percent germination and speed to give a more balanced view of germination. In this index formula, more weight is given to seeds that germinate earlier than seeds that germinate later on. Therefore, a higher germination index value indicates a faster percentage of germination and speed [58]. For cowpea, soybean, sorghum, and sesame, the highest germination index values were obtained at mostly shorter steeping durations for many of the isolates, although statistical parity with values from longer steeping periods was sometimes observed. This implies that shorter steeping durations are likely sufficient for improved germination by these Bacillus species. However, for okra, the highest index values were not limited to the shorter steeping durations which is likely as a result of the hard seed coat. These means that a longer steeping period is likely necessary for okra.
The seedling vigor index is simply a product of the seedling height and final germination percentage. This index is a simple yet effective measure of plant biomass in a seed lot. Early vigorous growth is typically linked to improved yield [59]. For cowpea, the best vigor index values were obtained at 2 h or 3 for most of the isolates. Even when longer steeping periods posted the highest vigor index, as in the case of inoculums C and D, they were not statistically different from values obtained at shorter steeping periods. This same cowpea pattern was observed for other crops.
Similarly, cowpea seeds bio-primed in Pseudomonas fluorescence for 6 hours and dried to original moisture content produced an improved vigor index [43]. In this study, cowpea seeds became bloated at longer steeping durations (4 and 5 h), and this negatively affected germination and consequently resulted in stunted growth, which ultimately produced low vigor index values. The subsequent drying of seeds in conventional priming ensured this difference between the optimal steeping durations obtained in this study and the one highlighted above. Bacillus megatarium was found to have a positive effect on the seedling vigor index of soybean after 8 days of sowing [60].
Overall, a relatively short steeping duration is required for bacterial priming of cowpea and soybean based on the vigor index data in this study. The vigor index measures plant biomass which is closely related to plant productivity. Again, the limited impact of biopriming time with these Bacillus species on the vigor index values of sesame and sorghum could be due to their unique structures. Also, okra is noted for its slow, erratic, and non-uniform germination pattern due to its seed hardiness. Therefore, a much longer steeping period than employed in this study may be appropriate for these three crops in bacterial priming.
Initial inoculum concentration of the isolates did not seem to show a consistent trend with final percent germination and mean germination time for all the crops. This means that the priming potential of the isolates is not directly linked to their concentration for these two parameters. This is not unsurprising since blotters have a low abundance of competing bacterial species, so a higher concentration is not necessary for effective colonization and promotion activity. Apart from this, bacterial species are capable of rapid proliferation under the right conditions, therefore the use of very higher concentrations may be superfluous. On the contrary, other priming methods involving the use of chemicals typically shown a concentration-dependent mode with typically drastic and deleterious effects at higher concentrations [60, 61].
Inoculum concentrations of the isolates did not seem to affect germination index values for sorghum, sesame, and okra. On the contrary, a mountain-shape trend for germination index was observed for isolates A and B for cowpea and soybean and isolates B and E for soybean alone, and isolate C gave a mountain-shaped trend for cowpea. Although, in some of these cases, statistical parity between values from different concentrations was observed.
There was an increase in vigor index with increasing concentration up to C2 or C3 for isolates A, B, and E for cowpea and isolates A, B, and C for soybean before a general decline in values was observed. For cowpea, isolate C produced a downward trend with increasing concentration for cowpea. Statistical equivalence was also observed between different concentrations. This could mean that after a certain concentration level, a diminishing return occurs.
The reductions, which were mostly insignificant, in germination and vigor indices with increasing inoculum concentration observed for some isolates could be ascribed to the increasing concentration of some potentially harmful substances secreted by the isolates. Stamenov et al. [62] alluded to this possibility in their study in which they recorded reduced germination in Allium cepa involving the use of Bacillus spp. and Pseudomonas spp.
The mechanisms responsible for this priming may include imbibition of the active growth promotion substances secreted by the bacterial species present in the medium and/or by direct colonization of the spermosphere, an effect that confers the pioneer advantage on these organisms when the seeds are later sown since early colonization can give the organisms a significant head start. This colonization can also enable potential plant growth promotion potential to establish itself first in an area (creating a physical niche) and possibly antagonize deleterious species through the production of antimicrobial agents. Effective colonization ensures that plant growth-promoting bacteria are better able to positively impact plant development [63].
Mechanisms of growth promotion that have been identified for Bacillus species include nitrogen fixation, solubilization and mineralization of phosphorus and other nutrients, phytohormone production, production of siderophores, antimicrobial compounds, and hydrolytic enzymes, induced systemic resistance (ISR) and tolerance to abiotic stresses [64]. In the study, nitrogen fixation can be excluded because of the time required for the nitrogen fixation process to be set up in plants and, also, solubilization and mineralization of phosphate that cotton wool was used as blotters and not soil, which can typically contain insoluble and organic phosphates that can be solubilized or mineralized.
CONCLUSION
To summarize, optimization of priming parameters is seldom attempted with bacterial paraments. The results shows that different seeds behave differently to bacterial priming. This study highlights the importance of steeping duration for seeds with large, hydrophilic endosperms such as cowpea and soybean during bacterial priming. For sorghums and sesame, cereals with small endosperms and impervious seed structure, the results were varied and steeping duration seemed to produce minimal impact on them. Also, the impervious nature of the seed coat of okra necessitates the use of higher steeping durations in future studies with bacterial priming. While chemical priming can have drastic and negative impact on growth parameters at high concentrations as reported by other studies, effect of changing inoculum concentration seems to be more subtle in this study. A full statistical optimization of the parameters for biopriming can help scale down the use of environmentally unfriendly options such as chemical primers. Further research under soil conditions will help in this regard.
LIST OF ABBREVIATIONS
| PGPB | = Plant growth-promoting bacteria |
| FGP | = Final germination percentage |
| MGT | = Mean germination time |
| GIX | = Germination index |
| VIX | = Vigor index |
RESAERCH INVOLVING PLANTS
The seeds were identified and authenticated at the Herbarium of the Department of Plant Sciences, University of Ilorin, Kwara State, Nigeria and the following voucher numbers were obtained: cowpea (UICH/004/1491/2022), soya bean (UILH/003/1490/2022), sorghum (UILH/002/1489/2022), sesame (UILH/005/1492/2022), and okra (UILH/001/ 1488/2022).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to Afe Babalola University, Ado-Ekiti, Nigeria, for providing the resources and facilities for the study.


