RESEARCH ARTICLE
Effects of Indigenous and Commercial Rhizobia on Growth and Nodulation of Soybean (Glycine max L) under Greenhouse Condition
Mulugeta Desta1, Ayele Akuma2, Metadel Minay3, Zekeria Yusuf1, *, Kassa Baye1
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e187407072302070
Publisher ID: e187407072302070
DOI: 10.2174/18740707-v17-230223-2022-17
Article History:
Received Date: 08/09/2022Revision Received Date: 19/01/2023
Acceptance Date: 25/01/2023
Electronic publication date: 06/03/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Soybean (Glycine max L.) is the most vital grain legume crop rich in protein and oil. The inoculation of rhizobia with soybean plays a very important role in increasing soil fertility via its contribution to biological nitrogen fixation.
Objective:
This study focuses on the evaluation of indigenous and commercial rhizobia on soybean nodulation and growth parameters.
Methodology:
Soil samples were collected from five districts for nodule trapping. The rhizobia were isolated using ‘plant induction following the standard procedures. The greenhouse experiments were arranged in a completed randomized design with three replications and two control units. The data were collected for plant height, nodule number, nodule dry weight, shoot dry weight, root length; root dry weight, total nitrogen and nitrogen-content.
Results:
The entire isolates were found gram-negative, without absorbing congo-red and did not grow on peptone glucose agar media. Slow grower isolates turned bromothymol blue with yeast extract mannitol agar medium into a moderately deep blue color but fast grower changed to yellow color. All isolates were tested on the sand induced nodule and were significantly superior to the negative control in terms of plant height, shoot dry weight, and nodule dry weight. The shoot dry weight of soybean rhizobial isolates on the sterilized sand experiment was ranging from 1.6 to 2.2g per plant and it was a highly significant correlation to the nodule number, nodule dry weight and root dry weight.
Conclusion:
The indigenous isolates were highly competent to all commercial rhizobia. This study encourages further evaluation of the field and molecular characteristics.
1. INTRODUCTION
Soybean (Glycine max L.) is the most vital grain legume crop in the world in terms of overall production and international trade [1]. It originated from the North-eastern China and cultivated worldwide under various climatic conditions [2]. Soybean is a protein and energy-rich crop and has nutrient requirements, especially phosphorus in adequate amounts and which is an essential element for legume growth. Soybean contains higher protein (40%) than other food crops and is second in terms of oil content (20%). The soybean seeds contain 23% carbohydrates, 4% fiber, vitamins, and minerals such as K, Mg, Ca, Zn, Fe and Cu as well as anti-oxidants [3]. The soybean responds well to inoculation with rhizobia and plays a very significant role in natural resource management due to its contribution to biotic nitrogen fixation [4].
Rhizobial biofertilizer from certain strains of valuable soil microorganisms cultured in the laboratory and packed in a carrier or without a carrier [5]. In Southern and Eastern African countries, microbial inoculants have been produced for different pulse crops since the early 1960s, with a radical increase as a function of time [6]. Soybean utilizes two roots of nitrogen for their growth through the soil and atmospheric Nitrogen (N2) that is fixed naturally in nodules formed in symbiosis with different species of rhizobium. The nitrogen fixation process is influenced by the existence, population and effectiveness of rhizobia, availability of nitrogen in the soil, plant genotype, age, plant-rhizobia interactions, and changes in soil physicochemical conditions [7].
Soybean can be successfully nodulated by strains of all the recognized species of rhizobia. The contribution of rhizobia to the nitrogen economy of the soil is quite substantial. The application of commercial rhizobial strains became widely known in parts where native nodulation is inadequate. Rhizobia inoculation has become an important agronomic practice to ensure adequate nitrogen supply to legumes to reduce the amount of inorganic nitrogen fertilizers required [8]. The use of commercial rhizobial strains of soybean has been universally known and is expected to be actual upon newly hosted varieties. Determination of the effectiveness of native rhizobium against commercial growth and nodulation of soybean is a vital aspect in increasing our validation of this biological process.
2. MATERIALS AND METHODS
2.1. Sampling and Preparation Techniques
The soil samples were gathered from five sites in the Arbaminch district, Ethiopia, for nodule taping. The commercial strains were obtained from the Holeta Agriculture Research Center and Menagesha Biotech Industry PLC. Soil samples were collected randomly in a zigzag fashion from ten soil cores at the depth of 0-20cm on the farmers' field at a 5m distance interval and combined to form a composite. A similar protocol was used for each soil collection site. The soil was smashed into small crumbs and thoroughly mixed, air dried and sieved using a 2mm sieve. The 10 g soil sample was diluted with 25 ml deionized distilled water. About 25 ml of the deionized water was added into the soil subsamples each weighing 10g and stirred for one minute. After equilibrating for 2-3hr, the suspensions were filtered and the pH was measured.
2.2. Design of the Study
This study was designed in complete randomized in 3 replications. The experimental study consists of twenty-four sterilized sand and twelve unsterilized soil treatments. The experimental treatments contain positive and negative controls. The nitrogen application was used in the form of KNO3. The seeds were externally treated and grown in each 3kg sand/pot and soil/pot. The effective soybean rhizobia and three commercial soybean rhizobia were tested on sterilized sand in an unsterilized soil experiment. The soybean seedlings were irrigated daily and maintained to grow until data collection.
2.3. Nodule Collection and Isolation of Rhizobia
Five soybean seeds were sown on each soil sample collected from each district in a sterile plastic pot containing a 3 kg capacity. The seedlings were watered to maintain moisture for 60 days or 50% flowering time. The plants were uprooted from the pots, and the intact, pink, and then larger nodules were detached from the main root for ease of handling to be transported through a vial containing silica gel until the isolation of rhizobia was undertaken at the laboratory level. The obtained nodules were carefully cleaned using distilled H2O to remove gross surface contamination. After an overnight soaking in distilled water, the nodules were again immediately immersed in 70% ethanol and 3% H2O2 for 3 minutes and 1 minute, respectively. The superficially treated nodules were cleaned 10 times with dH2O to eliminate the sterilants completely. Nodules were fragmented using a sterile glass rod in 0.1 N NaCl with drops of clean distilled water. One loopful of the nodule suspension was streaked on a YEMA plate containing 0.0025% Congo red with pH 6.8 in glass Petri dishes. The plates were inverted and protected at 28 °C for about 7 days [9].
2.4. Preparation of Culture Media
The YEMA-Congo red was prepared and used to cultivate indigenous rhizobia. The preparation of all media was conducted aseptically following standard methods. The medium was sterilized by autoclaving at 121oC for 15 minutes. The resulting autoclaved media were cooled then, poured onto cleaned petri dishes, and used for cultivating rhizobia.
2.5. Purification and Preservation of the Isolates
The single colony was used to transfer into 10 ml of YEM broth in a test tube, vortexed, and placed on a rotary shaker at room temperature for 48 hrs. A loopful of culture suspension from each tube was streaked on a sterile YEMA plate and incubated at 28°C for 5-7 days. Repeated sub-culturing was conducted until quality and consistency were sustained. Pure colonies were transferred to YEMA slants with 0.3% (W/V) CaCO3 until sufficient growth was observed and the slants were stored at 4°C.
2.6. Presumptive Test of the Isolates
Presumptive tests were done by re-culturing the primary isolates into YEMA containing Congo red and peptone glucose agar test then kept in dark conditions [10]. YEMA containing 25 µg, Congo red (10 ml L-1) was used to differentiate the isolates from Agrobacterium and to evaluate their abilities to absorb the dye. The bacterial strains were observed morphologically and typical rhizobia were gram-stained to confirm the purity of the culture. The inoculated medium was incubated in dark.
Peptone glucose agar was prepared using 20 g/L peptones, 10 g/L glucose, 5 g/L NaCl, 15 g/L agar, and 1L distilled water adjusted at pH 7.0. The assay was conducted to determine the ability of the isolate to utilize glucose as the sole carbon source for its growth. Growth interpretations were engaged after 15 days of incubation [11]. To determine the isolates using gram staining; a few drops of H2O was placed on a clean slide and culture was taken from the pure culture using a sterile loop and touched with the loop until it becomes turbid. The suspension was spread to form a thin film over the entire slide and air-dried. The heat-fixed smear was placed on a staining rack using crystal violet and iodine was added after 1 min. Alcohol (95%) was used to decolorize for about 30 seconds. The entire film was covered with safranin for 1 minute. Excess safranin was washed using distilled water. The cells of the isolate were then examined under the microscope [12].
2.7. Morphological Characterization and Biochemical Test
A loopful of test isolates from 48 hrs old YEMA broth culture was inoculated into a YEMA plate. The colony size, shape, texture, and color were characterized and recorded after 5-7 days of growth time. Isolates were incubated into YEMA-BTB (0.25% Bromothymol Blue) medium to determine the inoculum ability to produce acid or base and change of medium color. The formation of blue color was indicating an alkaline reaction on Bromothymol Blue whereas a yellow color indicates acid production in the medium [13]. To determine the catalase test a small culture was transferred on a clean slide with the help of a sterile loop. A 3% of H2O2 was added to the culture. The formation of air bubbles was considered as the occurrence of catalase enzyme which was produced by Rhizobium species as indicated by Singleton et al. [14].
2.8. Physiological Test
The physiological test of the soybean rhizobium isolates was conducted to determine the ability of salt, temperature, acid or base tolerance, and phosphate solubilizing. The isolates were tested for their tolerance to salinity in YEMA medium supplemented with NaCl at different concentrations (0.1, 0.5. 2, 4, and 6% (w/v) NaCl [15]. The capacity of the isolates to develop at several pH was verified on YEMA attuned at pH 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, and 9.0 using 0.1N HCl and 1N NaOH. The outcomes were noted qualitatively after 5-7 days of incubation at 28°C. The temperature tolerance of the isolates was measured at 10°C, 15°C, 25°C, 35°C, 40°C, and 45°C on the YEMA medium. The growth was quantified as (+) for colony growth and (-) for no colony growth as described by Alexandre et al. [16]. The potential of isolates to solubilize phosphate was tested by inoculating them on Pikovskaya agar medium containing glucose (10 g/l), Tricalcium phosphate (5 g/l), NH4(SO4)2(0.5 g/L), Yeast extract (0.5 g/L), Magnesium sulfate heptahydrate (0.1 g/L), Sodium chloride (0.2 g/L), Manganese sulfate (0.002 g/L), Ferrous sulfate (0.002 g/L) and Agar (15 g/L) at pH of 6.8. The phosphate solubilizing potential of the rhizobial isolates was confirmed upon the presence of a clear zone around the colonies [10].
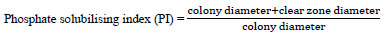 |
2.9. Symbiotic Effectiveness of Soybean Rhizobia
Fine-graded river sand was washed under tap water and immersed in 98% sulfuric acid for two days. After washing several times under distilled water to get rid of the last traces of the acid, the sand was autoclaved at 121°C for 15 minutes before being filled into surface-sterilized plastic pots [17]. The seed of soybean was treated in 70% ethanol and 3% H2O2 to remove external microbial contamination. The pot surface was sterilized using 95% ethanol. Pre-germinated soybean seeds were soaked in 0.75 (w/v) clean H2O and kept at 25 °C for 3 days and then transplanted into each plastic pot. Three plants were maintained in each pot. Three ml of the active culture of each isolate grown in YEMB was poured onto each seedling at germination time. The experimental setup was replicated three times and laid out in a complete random design. There were negative and positive control treatments. The negative control lacked both sources of nitrogen while the positive control was supplemented with 70 ppm which was added as 0.05% KNO3 (w/v) solution week-1.
The seedlings were watered using distilled water and all pots were fertilized with nitrogen-free nutrient solution. Seedlings in all replications were carefully uprooted to expose the whole root system after sixty days of planting. The adhering sand was removed by washing the roots with water over a sieve. Important parameters like nodule color, nodule number, dry weight, shoot dry weight root length, root dry weight, and relative synergetic success were used to confirm the candidate isolate for evaluation of symbiotic effectiveness of the isolates under sterilized sand conditions. Five representative nodules were taken from the same sample and dissected with a blade to observe their color in the center. The color score was made on 1-4 scale as: 1 = white, 2 = pink, 3 = slightly dark red and 4 = deep dark red [18]. The total numbers of nodules in all the uprooted plants of each replication were counted carefully. Fresh nodules were obtained after several nodules were pooled together including the dissected nodules for color determination. The number of nodules was averaged per plant and their dry weight was measured by drying at 70 °C to constant weight. The nodule dry weight was reported as mg plant-1. All the plants under the same replication of each treatment were positioned in a paper bag and oven-dried at 70 °C to constant weight. The average dry weight of the seedling was taken as dry weight per plant and reported as g plant. Finally, the percent of relative symbiotic effectiveness (RSE) of the isolates was computed to select effective isolates as:
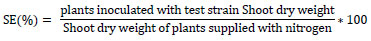 |
Where: RSE (%) values were rated as: >80% = highly effective, 50-80% = effective, 35-50 = lowly effective, and <35% = ineffective [19].
A greenhouse pot experiment on unsterilized soil was conducted for the evaluation of the most effective isolates found under the pot experiment on sterilized sand. The selection of the strain was conducted on their symbiotic efficiency percent values.
2.10. Soil Sampling and Analysis
The composite soil sample was collected at 0-30 cm depth from Rare Research Site, Haramaya University. The composite soil samples were uniformly mixed, by removing any debris and filling 3 kg soil into plastic pots while 1kg composite soil samples were used for the determination of selected physicochemical properties after being air-dried, ground, and filtered using 2 mm and 0.5mm sieve size. The unit size distribution (Bouyoucus Hydrometer Method), organic carbon (wet oxidation/dichromate digestion), total nitrogen (modified micro-Kjeldahl procedure), available P (Olsen extraction method), soil reaction or pH (1:2.5 soil to water ratio suspension), electric conductivity (1:2.5 soil to water ratio extract) and cation exchange capacity (ammonium acetate extract) were made following the standard analytical procedures of the respective parameter compiled [20].
2.11. Enumeration of the Indigenous Rhizobial Population
The plant infection MPN method was employed according to Somasegaran et al. [10] to determine the number of viable and infective native rhizobia in the soil. The soil was serially diluted starting from 10-1 to 10-8. The first dilution was made by adding 9g of soil into 100ml of distilled water and mixed thoroughly on a shaker for 10 minutes to disperse the soil. Likewise, the rest of the dilution was prepared serially by adding 1 ml of suspension in 9 ml of sterilized distilled water. Soil dilutions were inoculated aseptically by adding 1 ml of the soli aliquot on each of the four replicate test plants per dilution and labeled accordingly. The soybean seeds (Gazella variety) having uniform size were used for the experiment. The soybean was sterilized using 70%(v/v) ethanol for 3 minutes and transferred to a solution of 3% H2O2 for 3 minutes. The soybean was treated using distilled water in five changes and sown on small pots containing sterile sand. The plants were kept supplied with adequate distilled water as per need and supplemented with nitrogen-free nutrient solution per week. After four weeks, the number of rhizobia in the soil was determined using the MPN plant infection technique. The most probable number was calculated from the most probable number tables. The calculation was made using the mathematical formula:
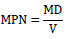 |
Where M = the most likely number from the MPN table for the lowest dilution of the series,
D = Lowest dilution (the first unit used in the tabulation)
V = the amount of aliquot used for inoculation.
2.12. Evaluation of Indigenous and Commercial Soybean Rhizobia
The assessment of symbiotic efficiency of the most relatively effective isolates was tested on unsterilized soils. The raw carrier material was crushed using mortar and pestle, sieved with a 0.5 cm mesh screen, and dried in a hot air oven at 60oC for two days. The materials were autoclaved at 121oC for 15 min. The powdered carrier base inoculants were prepared through the mixing of 5-7 days old isolate suspension (108 cells) and sterile charcoal powder in a 1:1 ratio (v/w). Healthy and surface-sterilized seeds of soybean were sown after being uniformly coated with powder inoculants of 1:1 thick slurry of 10% sugar solution in cool and sterilized water at a rate of 7g inoculants/kg seed and then immediately planted into irrigated pots. These pots were arranged in complete random design with two control treatments: the negative control without nitrogen and the positive control was fertilized with 70 ppm nitrogen added as 0.05% KNO3 to soybean plants in the greenhouse. The purpose of nitrogen fertilization was to measure the biomass yield potential of soybean in the experiment. Plant height was measured in centimeters from the ground level to the top of the plant at flowering plants from each plot. Data on nodule number, nodule color, nodule dry weight and shoot dry weight were collected. Shoot and root dry matter was measured at the mid-flowering stage of the crop from plants that were sampled for nodulation. Root length was measured in centimeters from the ground level to the tip of the root at flowering plants from each plot. The samples were placed in labeled perforated paper bags and oven-dried for 48 hours at 70oC to constant weight as described by Williams et al. [21] to determine the shoot and root dry matter. Qualitative determination of nitrogen was made as per the modified Kjeldahl method.
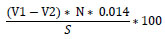 |
Where V1: ml of titrant (H2SO4) used for the sample; V2: ml of titrant (H2SO4) used for the blank; 0.014: meq weight of nitrogen in gram; S: weight of the sample; N: Normality of the H2SO4; mcf: Moisture correction factor.
Total N uptake was calculated as N content
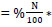 * Shoot dry weight
* Shoot dry weight
2.13. Data Analysis
The collected data of plant height, shoot dry weight nodule number, nodule dry weight, shoot dry weight, root dry weight length percent nitrogen and nitrogen content were analyzed by analysis of variance using the general linear model procedures of SAS version 9.2. A least significant difference test was used for separating the significant mean at P < 0.05. Pearson’s correlation coefficient (r) was determined to confirm the direction and magnitude of the association between the studied parameters.
3. RESULTS AND DISCUSSION
3.1. Rhizobium Species Identification
The presumptive tests were conducted by re-culturing the primary isolates on YEMA containing Congo red and peptone glucose agar test medium. The obtained rhizobia isolates were gram-negative, rod-shaped bacteria that did not absorb Congo red from the YEMA-CR medium and no growth was observed on peptone glucose agar. Out of the 22 isolates, only 4 of them (SBGDF3, SBGDF5, SBCH4, and SBMS1) were slow growers on the YEMA- BTB medium and showed the blue color which indicates the acid-producing ability of the isolate. However, the remaining 18 isolates (SBDF1, SBDF2, SBDF4 SBDF6, SBCH1, SBCH2, SBCH3, SBMS2, SBOL1, SBOL2, SBDS, SBDS2, SBKF1, SBKF2, SBKOF3, SBGO1, SBGO2, and SBGO3) were fast grower and changed the medium from deep green to yellow color within 3-5days. The yellow color of the bromothymol blue medium was obtained due to the alkali production of the fast-grower rhizobia. Colony size, shape, margin, elevation, texture, and color were determined on the YEMA medium.
The colony diameters of all isolates were within the range of 2.0 mm to 5.5 mm. The smallest colony was shown by isolates SBCH4 which was collected from Arba Minch Zuria whereas the largest colony (SBOL2) was isolated from the Mirab Abaya district. Based on colony morphology, isolates were categorized as large mucoid, typically convex and white colonies. The shape, margin, and color of most of the newly obtained strains were circular, smooth, and white. But few of them were flat in elevation and buttery in texture, and others were raised and elastic. Temperatures ranging from 10°C to 45°C were investigated to determine the influence on the growth of isolates. The growth of isolates was observed at all temperature ranges except 10°C and maximum growth was observed between 20°C to 30°C. All the isolates were tolerant to the pH between 4-9, slow grower isolates grew denser near pH4 and scattered at pH 9 when compared to the fast grower. All isolates survived a salt concentration of 5%. However, some of the isolates (SBDF1, SBDF2, SBDF3, SBDF6, SBMS2, SBDS2, SBKF1 and SBKF3) were grown on NaCl concentration of 6%.
3.2. Symbiotic Effectiveness on Sand Culture
All tested isolates on soybean were able to form nodules in a pot experiment of sterilized sand culture under greenhouse conditions. Inoculated soybean showed a significant (p <0.05) increase in all parameters when compared to the negative control treatment but no statical difference between indigenous rhizobia inoculants and the positive control on the plant height and root length parameters. The plant height was measured from 16 cm to 40cm and statistically showed a positive correlation with the nodule number as well as a highly positive correlation with nodule dry weight shoot dry weight, root length, and root dry weight. The negative control displayed a highly significant (p<0.05) short plant height when compared to the other treatments, while the positive control scored a high plant height which is significantly different (p<0.05) compared to the SBMS1 and SBDS1 indigenous isolates. The soybean plant inoculated with the SBOL2 isolate identified in the soil sample collected from Mirab Abaya numerically gave the maximum nodule number of 33.6 per plant followed by the nodule number 29.3 per plant from Demba Gofa. The minimum nodule number obtained during the treatment was 7.3 SBDF1 from the ArbaMinch Zuria district. The shoot dry weight of soybean rhizobial isolates on the sterilized sand experiment was ranging from 1.6 to 2.2g per plant (Table 1).
| Parameters | pH | OM(%) | TN(%) | OC(%) | P(mg/kg) | Particle size (%) | Texture class | PMN | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||||||
| Value | 6.12 | 0.99 | 0.14 | 0.57 | 18.52 | 72 | 14 | 14 | Sandy loam | 1.7 |
The SBOL2 rhizobial isolate had a significantly important (p<0.05) relative to the negative control and it was a highly significant positive correlation relation with the parameters of plant height and root dry weight. Analysis of variance indicated that inoculation had a significant effect (p<0.05) on root length when compared to the negative control treatment and it was highly correlated with plant height and root dry weight (Table 1). The root dry weight of the inoculated soybean in the sterilized sand experiment was highly positively significantly correlated (p<0.05) with plant height, root length and shoot dry weight. In the current investigation, the symbiotic effectiveness has scored a minimum of 70% and a maximum of 96%, from the total isolates 63.6% of isolates showed very symbiotic effectiveness.
3.3. Soil Properties and Population of Indigenous Rhizobia
The soil physicochemical properties and the indigenous soybean-infected rhizobia were determined before sowing soybean seed on the unsterilized soil experimental pots. The available phosphorous and total nitrogen was 18.52 mg/kg and 0.14, respectively (Table 2). Seven effective isolates (SBDF1, SBDF2, SBDF6, SBCH1, SBOL2, SBKF2, and SBGO2) and three commercial strains were evaluated on the unsterilized soil.
The indigenous SBCH1isolates and MAR 1495 were highly significant (<0.05) in the plant height when compared with all treatments except SBCH1, MORDOK, and positive control. The plant height had a highly significant correlation (p< 0.05) with nodule dry weight and shoot dry weight but a negative correlation with the root length of the soybean. The nodule numbers of the indigenous isolates were counted from 8 to 27 indigenous isolates and commercial isolates from 24 to 28 while the nitrogen application treatments had 5 nodules per plant (Table 3).
In this study, newly isolate indigenous rhizobial and commercial strains involved as inoculants of soybean were used. The obtained isolates were recultured on YEMA containing Congo red and peptone glucose agar authorizing that the isolates were rhizobium strains. A similar study was made by Ali et al. [22] rhizobium strains incapable to absorb Congo red stain in the YEMA CR medium. The fast-growing rhizobial isolates on YEMA plates containing BTB were identified by showing yellow color due to alkali production and the slow-growing isolates were shown a blue color and production of acid. The fast-growing and slow-growing strains were either poor or had no growth on peptone glucose agar medium; this might confirm that the isolates were rhizobia strains [23]. In the present study, the nodule color was shown as 63.6% pink, 22.7% slightly dark red, and 13.7% deep dark red (Table 1).
Astuti et al. [24] found pink, slightly red, and deep dark red internal nodule colors of effective and highly effective soybean isolates whereas white colors were from the non-effective ones. Waswa et al. [25] also stated that effective nodules were marked by reddish color due to the formation of Fe-rich hemoglobin that regulates the oxygen content, thus promoting nitrogen fixation activity. The shoot dry weight ranged from a minimum of 2.6 to a maximum of 4.5g in the indigenous rhizobia isolates. In the same line, Daniel et al. [26] found that the maximum shoot dry weight of 4.1 g /plant when inoculated with the commercial strains treatments and only 1.37 g/plant in the un-inoculated treatment. All treatments did not show a significant variation in parameters of root length and root dry weight (Table 3). Root length without inoculation was longer than root length with inoculation although the root length, of inoculated soybeans, had a higher root dry weight. Plants that lack a nutrient try to meet it by extending the roots [27]. The quantity of nitrogen content of a single soybean plant at the negative control treatment had a less significant value when compared to the other treatments (Table 1). The shoot nitrogen content of the plants ranged from 0.8% to 3.3% strain 532c [26]. The indigenous rhizobia isolates had shoot dry matter yields ranging from 1.6 to 2.2 mg per plant in the sand experiment. This result was higher when compared to another study [28] where shoot dry weight between 0.6 and 1g plant-1. According to Saharanand Nehra [29] reported the maximum availability of phosphorus usually occurs in a pH range of 6.0 to 7.0 and it is essential for the general health and vigor of the soybean plant.
4. CONCLUSION
Rhizobia inoculation plays a very important role in biological nitrogen fixation on the legume plants by increasing the nitrogen nutrient and the yield of the crop. The selection of indigenous isolates using their symbiotic effectiveness and evaluation with commercial strain is very crucial. Using commercial rhizobial inoculants has been widely recognized. However, it is also vital to assess the effectiveness of indigenous strains against commercial ones. This is a preliminary finding to define the importance and affordability of indigenous isolates against commercial strains. Greenhouse trials were arranged in three replication and two control units. The data of plant height, nodule number, nodule dry weight, shoot dry weight, root length; root dry weight, total nitrogen%, and nitrogen -content were statistically analysed. All the isolates were gram-negative and did not absorb Congo-red on YEMA-CR media and growth was not observed in peptone glucose agar medium. Rhizobia strains inoculation would be vital progress to nitrogen fixation in increasing the production of several crops. This study might encourage further field investigation and molecular characterization of the isolates to explore the potential of indigenous isolates on the growth and nodulation of soybean.
AUTHORS’ CONTRIBUTIONS
Mulugeta Desta and Ayele Akuma: outset and designed experiments. Kassa Bayeh: performed experiments. Zekeria Yusuf: contributed reagents and materials. Metadel Minay: performed all the experiments and data analyses and wrote the manuscript. All authors discussed and revised the manuscript. All authors commented on the manuscript before submission. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data will be available on request to the author [Z.F].
FUNDING
This project was funded by a Haramaya University Research grant, under project code: HURG_2021_06_01_22.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This work was supported by grants from Haramaya University and Southern Agricultural Research Institute, South people Nation and Nationalities Regional state, Ethiopia.








