All published articles of this journal are available on ScienceDirect.
Preliminary Characterization of a Functional Jam from Red Chicory By-Product
Abstract
Background:
The by-products of red chicory leaves are a valuable source of bioactive compounds that can be exploited in the development of functional foods.
Objective:
This work aimed to combine healthy properties of red chicory by-products with other ingredients in the formulation of a functional jam, which is easy and safe to swallow, especially for people suffering from dysphagia.
Methods:
The physicochemical parameters, as well as the total polyphenols content (TPC), was assessed in the obtained product.
Results:
The TPC (549.44 mg GAE/100 g) was higher than the values reported in other jams, and it remained stable along with the colour during six weeks of storage. Within the carbohydrates, 0.4% of the prebiotic fibre inulin has been detected, suggesting that this jam formulation is a promising delivery system of phenols and fibre. From the sensorial point of view, the functional jam obtained an overall good acceptability judgment. The bitterness of the red chicory is persistent, which helps people with dysphagia swallow more easily.
Conclusion:
The functional jam, based on chicory by-products, could be a good source of bioactive compounds, which are helpful even in the disabled subjects’ diet.
1. INTRODUCTION
Cichorium intybus L. belongs to the Asteraceae family, and it is commonly named chicory. This plant is of great commercial interest, and its farming is widely distributed in Asia, North America, and Europe. Almost 8,500 ha are cultivated in north-eastern Italy, with red chicory, and the harvested production is about 280,000 tons per year (ISTAT), where the cultivar Chioggia is the most representative variety [1, 2].
Besides its economic and culinary features, chicory is noteworthy for other reasons. Firstly, it belongs to traditional medicine since all parts of this plant contain potentially healthy bioactive compounds [3]. Chicory has been demonstrated to act as a prebiotic in animal models [4] on bifidobacteria and lactobacilli populations as a consequence of the concentration of fermentable fibre substrates. Inulin is present as a plant storage carbohydrate in more than 30,000 plant species, including chicory, where a range from 10.65 to 44.69% has been reported in leaves and roots, respectively [5]. Also, chicory is a source of antioxidant molecules [6], such as flavonoids, which were reported in the range 5-110 mg quercetin/kg, according to the chicory variety [7]. These bioactive compounds are described to protect the body not only from bacterial and viral infections but also from degenerative and age-related pathologies [8]. Antioxidant, anti-inflammatory, hypolipidemic, gastro-protective, and antidiabetic have been some bioactivities related to this plant [9]. In addition, the bitter compounds present in chicory such as sesquiterpene lactones have proved its antimalarial and anthelmintic activity [10] as well as its antimicrobial potential [11]. Therefore, the consumption and the production of chicory should be encouraged, as proposed by the Chicory Innovation Consortium [12]. From the circular economy point of view, it must be considered that chicory crops produce many by-products that might be valorised. It was calculated that residues (leaves, stems, etc.) constitute about 40-50% of the harvested material [13]. These by-products, rich in healthy bioactive substances, could be considered a promising ingredient for functional foods formulations. Jam is a type of food consumed by a large part of the population, from children to the elderly. In addition, the jam processing parameters could not only preserve several bioactive compounds such as total phenolics, flavonoids, and betalains but also improve their bioaccessibility [14]. Moreover, due to its particular texture, jam is a food administrable to long-term hospitalized people who frequently suffer from dysphagia, which is a disorder characterised by difficulty in swallowing, associated with several pathological conditions, including neurological disorders like dementia and stroke [15]. In this regard, dysphagia may result in severe consequences, such as nutritional and respiratory complications and even death.
To our knowledge, there is little information about the processing of red chicory by-products within the food industry. Therefore, this paper aims to carry out a preliminary characterisation and to assess the possible acceptability of an innovative functional jam formulated with red chicory by-products and other natural ingredients.
2. MATERIALS AND METHODS
2.1. Materials and Reagents
The red chicory (Cichorium intybus var. Chioggia) leaves by-products were collected by a local farmer in Legnaro (Padova, Italy), located in the Veneto region, and the Golden Delicious apple (Malus communis var. Golden Delicious), lemon, and pineapple were purchased from a local supermarket in the same location. All chemicals and solvents were of analytical grade and purchased from Sigma (Sigma Chemical Co., St. Louis, MO, USA).
2.2. Jam Formulation
Red chicory pinstripes (about 10 cm in length and 1 cm in width) were mixed with apple cubes of 1 cm per side. They were blanched (2 minutes at 75 °C) in water containing 0.2% w/v of citric acid and 0.2% w/v of ascorbic acid. Lemon juice was obtained by manually squeezing the fruits. Pineapple juice was extracted by cutting the fruits in two halves, eliminating the peel, and recovering the juice in a centrifugal juicer (Moulinex JU655, Group SEB, Ecully, France). Apple food-grade pectin and sugar were added at the concentration reported in Table 1. The jam obtained was stored for 6 weeks at 10 °C in glass containers.
| Ingredients | Proportion (%) | Weight (g) |
|---|---|---|
| Red chicory | 29.2 | 146 |
| Apple | 29.2 | 146 |
| Sugar | 30 | 150 |
| Pineapple juice | 7 | 35 |
| Lemon juice | 3.5 | 17.5 |
| Pectin | 1.87 | 9.35 |
| Total | 99.9 | 500 |
2.3. Water Activity (aw), Total Solid Soluble, and pH Measurement
After the centrifugation (2370 g at 4 °C for 10 min) of the sample, the aw, the total solid soluble as Brix degrees, and the pH value were detected in the red chicory Jam Supernatant (JS) by using the Lab Master-aw instrument (Novasina AG, Lachen Switzerland) at 20 °C [16], the K71601 RM 45 digital refractometer (Optech) (AOAC 932.12) [17], and the potentiometer HI221 (Hanna) at 10 °C, respectively.
2.4. Stability of the Colour Along the Time
The colour (n = 3) of the jam along 6 weeks of storage was analysed weekly on a spectrophotometer (Minolta CM-508C). Before each series of measurements, the spectrophotometer was calibrated on a standard white tile, as reported by previous research [18]. The whole visible spectrum (400-700 nm) was recorded (∆λ = 2nm), and the illuminant was “natural light” D65/10°55,56. The results are expressed as L*(lightness, where 100 is a perfect reflecting diffuser and zero is black), a* (+a* is redness while –a* is greenness), b* (+b* is yellowness and –b* is blueness). Additionally, ∆E is the sum of the total changes, and it was calculated as follows: (∆L2 +∆a2 +∆b2)1/2.
2.5. Determination and Stability of Anthocyanins at Different pH Values
The total anthocyanin concentration in the JS was calculated at 535 nm, according to previous research [19], by using a calibration curve of cyanidin 3-glucoside and expressed in µmol/g. In addition, the JS was resuspended in a citrate-phosphate buffer solution (0.1 M citric acid and 0.2 M Na2HPO4) in order to assess the stability of the anthocyanins at pH values ranging from 3.0 to 7.0.
2.6. Total Polyphenols Content (TPC)
The TPC of the jam was determined as reported previously [20] and expressed as mg gallic acid equivalents/100g (mg GAE/100g). The TPC was monitored along 6 weeks of storage.
2.7. HPLC Determinations
The polyphenols were evaluated according to the method previously reported [21] by using the HPLC Thermofinnigan Spectra System UV6000LP with detector Diode array in the 200 to 600nm range. The LC-18 Supelco-sil column was used at the following operating conditions [22]: the mobile phase was a mixture of water acidified with sulfuric acid (pH 2.5) and methanol at different elution gradients and flow rates, at 40°C, and with a run time of 100 min.
The carbohydrates were determined by using the same HPLC system reported before and an Aminex HPX87-C anion exchange column [23]. The mobile phase was deionised, using 0.22 µm of filtered water, with a flow of 0.6 mL/min, and the time of analysis was 40 minutes at 80°C. In both determinations, the analysis was made by injecting 20 µL filtered samples from both Jam Precipitate (JP) and JS resuspended in 1:2 distilled water.
2.8. Acceptability Evaluation
In order to evaluate the overall acceptability of the chicory jam, a tasting test based on a consensus approach was used to allow further discussion on the jam samples. Ethical review and approval were waived for this study since the participation was voluntary. In addition, all data were anonymous.
The evaluation of the jam samples was performed in tasting booths, with controlled environmental conditions [24], by ten volunteers (students and personnel working at the laboratory, 6 females and 4 males within the age ranging from 22 to 55, media 30) identified as regular consumers of jams. Informed consent was obtained from all subjects involved in the study. The volunteers were instructed to define the descriptors “bitterness” and “colour” of the product. Based on these attributes, they were asked to determine the overall acceptability of the jam on a 5-point hedonic scale, where 1 corresponded to “completely refused” and 5 to “completely accepted.”
3. RESULTS AND DISCUSSION
3.1. Water Activity (aw), Total Solid Soluble, and pH Measurement
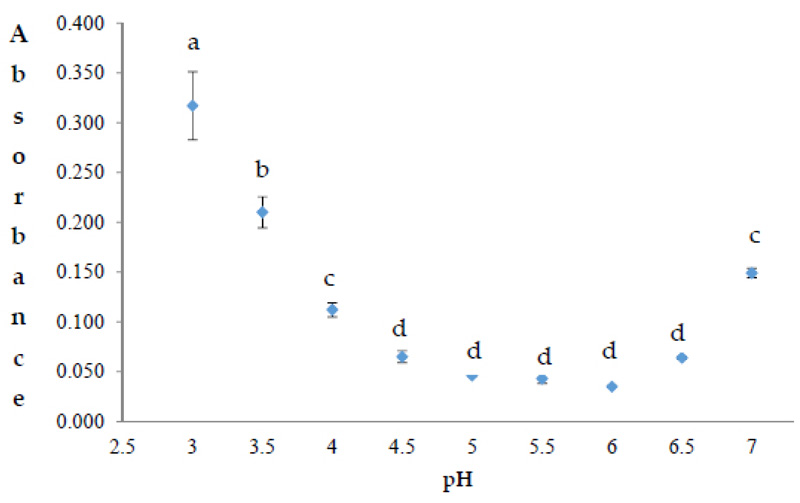
The aw, total solid soluble, and pH values of the jam are 0.91 ± 0.02, 3.61 ± 0.06, and 46.3 ± 0.03 Brix degrees, respectively. These data are in agreement with the values found in jams obtained from other by-products [25]. At the pH from this study (3.61 ± 0.06), the activity of polyphenol oxidase, which is involved in enzymatic browning and phenols nutritional value loss, slows down [21]. In addition, according to Fig. (1), the highest absorbance of anthocyanins was reported at pH 3.0, with these molecules being more stable at that value. Low pH amounts are related to a high extraction yield of bioactive compounds such as anthocyanins [19]. Therefore, the jam from this study has preserved the concentration of this bioactive compound, which was 1.25 µmol/g. The presence of ascorbic acid has an influence on the stability of anthocyanins [26]. Therefore, the presence of lemon juice in the jam may contribute in this regard. Anthocyanins have been responsible for the high antioxidant activity found previously in red chicory [27]. Several glycosylated forms of cyanidin (representing the main fractions of anthocyanins), such as cyanidin-3-malonyglucoside (177.3 µg/g) and cyanidin-3-O-glucoside (14.4 µg/g), were previously found in the red chicory variety Chioggia [13].
3.2. Stability of the Colour Along the Time
Table 2 shows the variation of different colour parameters along the time of the jam storage. There was a small increase in L* (p > 0.05), with the jam being lighter after 6 weeks than at T0, which is consistent with a previous report indicating that storage increases lightness [28]. In addition, the change in L* values has been related to the change in the jam consistency during processing [26]. A lighter colour could represent a loss of redness due to the degradation of the bioactive compounds during the jam processing since some of them are sensitive to heat [28]. However, the redness from this study was improved by 3.34%, as the a* value has increased (p > 0.05) after the sixth week. The presence and stability of anthocyanins and other phenols from the red chicory and the rest of the ingredients could impact the preservation of the redness, as mentioned in previous research [29]. Moreover, the initial values of a* and b* were similar to the ones reported previously in another jam from an underutilized fruit [30]. Regarding the ∆E (distance between two colours), this was not visually perceptible since a value of 1.42 was found, according to the literature [18].
| Week | L* | a* | b* | ∆E |
|---|---|---|---|---|
| 0 | 63.83 ± 1.46a | 7.19 ± 0.44a | 27.32 ± 0.97ab | - |
| 2 | 63.58 ± 2.40a | 6.50 ± 0.53a | 26.05 ± 1.22b | 1.47 |
| 4 | 65.87 ± 1.58a | 6.78 ± 0.47a | 26.66 ± 1.39ab | 2.18 |
| 6 | 64.87 ± 0.89a | 7.43 ± 0.53a | 28.25 ± 0.42a | 1.42 |
3.3. Quantification and Polyphenols Profile
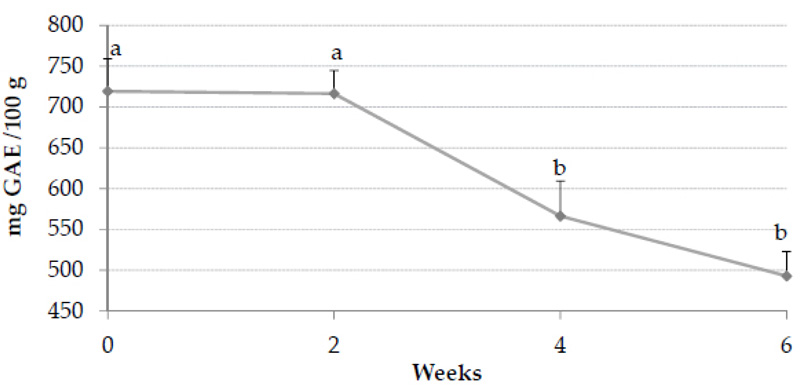
Polyphenols were other bioactive compounds detected at the end of the jam processing before the storage (T0). Six substances were detected (Table 3), and epigallocatechin-gallate had the highest concentration (23.78 ± 0.49 mg/L) of all the phenols assessed. Catechin, epicatechin, and epigallocatechin-gallate have been previously detected in red chicory (9). On the other hand, the content of chlorogenic acid (75.71 ± 0.73 mg/L) could be related to the presence of this phenolic acid in Chioggia red chicory variety [31], whose concentration in this plant is interesting for human food fortification [9]. Hypoglycaemic and hypolipidemic properties in chicory have been previously associated with the content of chlorogenic acid [32], while phenols are related to being suitable as diabetes and obesity modulators [33]. Besides red chicory, apples can contribute to the TPC since they have been reported to present epicatechins and catechins [34]. This finding could be of great interest from the nutritional point of view since those compounds are considered natural inhibitors of the enzyme α-glucosidase, which is involved in carbohydrate dysmetabolism [35]. The amount of phenolic compounds was reported to remain unchanged during the jam processing, preserving the bioactivity in the final product [36]. The TPC reported at T0 was 719.27 ± 39.94 mg GAE/100 g, while a significant decrease (p < 0.05) of the content of TPC was observed in the first four weeks of storage of the jam (Fig. 2). The reduction in the TPC could be explained by their function as protein glycation inhibitors and scavengers of dicarbonyls [37, 38]. In addition, the polymerization of phenols could be another explanation for the decrease in their concentration, resulting in high molecular and insoluble polymers [39]. In the following time, the polyphenols did not show a considerable reduction, and after six weeks of storage, the TPC obtained the lowest concentration (492.75 ± 30.18 mg GAE/100 g), in comparison to T0 (p < 0.05). However, this value was higher than the values reported in grape (449.2 mg GAE/100g), ground raisin (274.9 mg GAE/100 g), bilberry (450 mg GAE/100 g), and bilimbi (23.65 mg GAE/100 g) jams [30, 40, 41] in previous research. In addition, even the TPC at the end of the storage time was higher than the range previously found in chicory leaves from several varieties [7].
| Polyphenols | mg/L |
|---|---|
| Epigallocatechin | 23.78 ± 0.49 |
| Catechin | 0.83 ± 0.05 |
| Epigallocatechin-gallate | 134.78 ±1.15 |
| Epicatechin | 2.44 ± 0.02 |
| Epicatechin gallate | 26.66 ± 0.22 |
| Chlorogenic acid | 75.71 ± 0.73 |
3.4. Determination of Carbohydrates
Regarding the content of carbohydrates in the jam (Table 4), sucrose has obtained the highest value (31.80%) of the total sugars evaluated. This compound was added during the jam processing, and it is important for the pectin gelation [42], which could also be related to the high content of calcium of the chicory leaves, according to the literature (292.61 mg/100 g) [5]. Conversely, lower concentrations of sucrose have been reported in red chicory leaves (2.6-11.1%), where fructose and glucose are the main carbohydrates found, even in higher amounts than in other parts of the plant such as roots, seeds, and peel [9]. On the other hand, fructose and glucose have reached 4.6 and 4.4% of the jam, respectively, and the sum of the simple total sugars found in the jam (40.8%) is lower than those registered in the majority of commercial jams [43].
| Carbohydrates | g/L |
|---|---|
| Inulin | 3.99 ± 0.2 |
| Sucrose | 318.4 ± 0.3 |
| Glucose | 44.2 ± 0.4 |
| Fructose | 45.7 ± 0.2 |
The content of inulin detected in the jam was about 0.4% of the carbohydrates assessed. This content is in the range reported in chicory leaves [5]. Inulin has been recognized to present functional properties such as cholesterol-reducing ability [44], and it presents prebiotic characteristics since it resists digestion in the upper digestive tract, being hydrolysed and then fermented by colonic bacteria [45]. Moreover, the jam from this study reached 1.16% of total dietary fibre, based on the Nutrisurvey software [46]. According to the European Union legislation, the food label can mention “source of fibre” or “high fibre” if it contains at least 3 or 6 g of dietary fibre/100 g of product, respectively [47]. Despite the fact that the values found in the jam are low, according to the current legislation, supplementation with other inulin and fibre sources could be suggested in order to increase the concentration of this compound.
On the other hand, as far as we know, the supplementation of inulin has never been considered in jams before. Regarding the fibre added to the jam, the supplementation with inulin extracted from chicory roots could be suggested, as reported in a previous study in the elaboration of yoghurt [48]. Furthermore, promising by-products have already been employed to enrich food products in dietary fibre content, such as the water-extraction residue from maize milling as well as β-glucan fractions from barley and mushroom [49, 50].
3.5. Acceptability Evaluation
The bitter taste of phytochemical compounds or phytonutrients, such as antioxidants, is considered the main cause of the rejection of vegetables and their derivatives by consumers [51]. For this reason, the food industry and the breeders usually try to decrease the content of bitterness and thus healthy substances from their products [52]. In this context, chicory species represent a peculiar case since they are well-known for their mild bitter taste, which is usually appreciated by consumers [53]. In the case of raw chicory, bitter notes are attributed mainly to sesquiterpene lactones [54]. The specific bioactivity of these substances is related to their capacity to activate taste bitter receptors type 2 (TAS2R) family present in the mouth [55]. The chicory jam received an overall good acceptability judgment (about 4 on a scale from 0 to 5) from the volunteers (Table 5).
This fact may be related to the jam inulin content since this compound has been used in previous research to improve the sensory properties in a different food matrix [56, 57]. Moreover, in the evaluation of these preliminary results, it must be taken into account that within the chicory jam preparation, the cooking procedure, and the addition of sugar have partially hidden the original bitter taste of the raw vegetable, whose persistent bitterness [58] could be functional in subjects with dysphagia problems. A specific therapeutic program (called deep pharyngeal neuromuscular stimulation) based on the use of thickening lemon juice has been used to improve pharyngeal swallowing, and it is demonstrated that the stimulation of the taste buds of bitter and base of tongue improve tongue retraction [15].
| Volunteers | Sex | Age | Final hedonic judgement |
|---|---|---|---|
| 1 | Feminine | 23 | 5 |
| 2 | Feminine | 24 | 4 |
| 3 | Masculine | 25 | 5 |
| 4 | Feminine | 35 | 3 |
| 5 | Masculine | 27 | 2 |
| 6 | Feminine | 55 | 4 |
| 7 | Masculine | 22 | 5 |
| 8 | Feminine | 30 | 3 |
| 9 | Masculine | 25 | 4 |
| 10 | Feminine | 34 | 4 |
CONCLUSION
The present research has explored the possibility of obtaining a functional basilar jam containing about 30% of red chicory leaves by-products. Our preliminary data confirmed that this jam is a promising delivery system of wholesome bioactive compounds, such as polyphenols and inulin. Also, the total phenolic compounds have not presented a substantial change after 6 weeks of storage. A preliminary tasting test, indicating the good overall acceptability of the product, was performed. The functional jam, based on chicory by-products, resulted in food that could be useful even in the disabled subjects’ diet. The basilar recipe could be further functionally improved by using other ingredients, e.g., inulin, that could be recovered from other vegetable by-products as the chicory roots.
LIST OF ABBREVIATIONS
| FOS | = Fructooligosaccharides |
| GAE | = Gallic Acid Equivalents |
| JP | = Jam Precipitate |
| JS | = Jam Supernatant |
| TPC | = Total Polyphenols Content |
ETHICAL STATEMENT
Ethical review and approval were waived for this study since the participation was voluntary.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting findings of this study is present within the manuscript.
FUNDING
This work was supported by the University of Padova prot. DOR 2032990 and University of Verona prot. JPVR18ZW52.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are thankful for the skillful technical support of Stefania Zannoni, Federico Zocca, and Roberto Conter.


