All published articles of this journal are available on ScienceDirect.
Impact of Microencapsulation on Two Probiotic Strains in Alginate Chitosan and Eudragit S100 Under Gastrointestinal and Normal Conditions
Abstract
Background:
Nanoparticles in biotechnology studies have played a significant role during the recent years and a wide range of them are being applied in food industries to prolong the microorganisms viability for more effective function in food processing and human gut.
Methods:
The main purpose of this research was evaluating the viability of two bacteria of Lactobacillus casei and Lactobacillus bulgaricus treated through double-coated beads including alginate Chitosan (First coating), and Eudragit S100 (Second coating) in simulated Gastrointestinal (GI) circumstance and yogurt. Free cells were employed as a control test and the results reflected that microencapsulated strains can survive longer than the normal cells.
Results:
The number of free cells of L. casei and L. bulgaricus respectively decreased from 6.0×106 and 7.2×106 (In the first day) to 4.1×105 and 5.3×106 (In the day 32) in GI condition. Also, in the same intervals of time, the number of double-coated L. casei and L. bulgaricus decreased respectively from 6.9×108 and 7.1×108 to 4.5×107 and 3.1×107 in simulated condition. Furthermore, the pH rate steadily decreased, however, it was more dramatic in the first week, whereas the trend gradually became more moderate in the last two measurements.
Conclusion:
Results indicated that microencapsulation increases the bacteria viability. Also, the pattern of pH changes was similar for both strains and revealed that the rates of pH and acidity in both double-coated and normal forms are close to the control test in the final measurement.
1. INTRODUCTION
Nowadays, probiotic strains are recognized as the beneficial live microbial supplements in food products which incredibly improve the function of the intestine in human through cooperating with prebiotics. These valuable strains have a potential to be inserted in various sorts of food, drugs and nutritional supplements [1]. Some identified merits of probiotic strains on health are proven, as they are capable of reducing the cholesterol in serum, improving the immunity systems as well as increasing lactose consumption in individuals who would not be able to properly digest it [2, 3]. Generally, the scientists argue that “Probiotic foodstuffs” is given to products entails at least 107 CFU g-1 bacteria, while the consumer uses it. One of the salient examples of these kinds of foodstuff is the dairy fermented product, particularly cheese, buttermilk, and yogurt. Moreover, these microorganisms due to creating pleasant flavor and aroma have found their reputation among diverse levels of a community. As a result, dairy products would be considered as suitable carriers for probiotics to function perfectly [4, 5]. Undoubtedly. Lactic Acid Bacteria (LAB) are the most prominent strains of probiotics, in particular, Lactococci, Lactobacilli as well as Bifidobacterium species which live in the gastrointestinal tract. Not surprisingly, Lactobacilli have the high capacity to adhere to the cells called “Enterocytes” which make them as a feasible candidate among the other genera of LAB [6, 7].
As a result, there are certainly few characteristics which must be addressed in dairy products including physicochemical attributes, viability in the products and human body, especially in storage and processing stages and finally, desirable organoleptic properties. Occasionally, thermal stresses, being under osmotic pressure and acid treatments lower the quality and effectiveness of probiotic strains [8, 9]. Consequently, it seems that when these vulnerable strains are covered via a micro - capsulation method, they have far effective performance and extended existence in the gastrointestinal lumen and fermented products. In other words, this mechanism acts through creating a layer between the environment and bacteria cells [10]. Some earlier studies indicated that when bacteria are covered by “Chitosan” consequently, their viability is going to be increased, spontaneously. Since, this low-price and nontoxic substance is broadly being applied to the immobilization operation of microorganisms, it is recognized as a reliable material [11-13]. Briefly, Chitosan is classified as positive charge of polysaccharide which is produced from deacetylation of chitin. Additionally, this unique material effortlessly would be distributed in acid pH and has the ability to bond itself to various anions including Eudragit S100 (EU) which is the commercial name for Rohm GmbH; as finding itself in the global markets in the middle of 1950s [14, 15]. This product is a result of polymerization of acrylic and methacrylic acid. Overall, the EU is a kind of polymer nontoxic powder which has a broad range of solubility. In the beginning, it was used to be prevalent to be applied as a courtier in solid medicines namely granules, capsules and tablets, but today, it has differing functions. In the research mainly EU S100 would be used as a coater which is driven from methacrylic or methyl methacrylate and it is highly unlikely to be solute in water and acids in spite of solution at pH 7 and even higher. This is exactly a feature leading to have the ability to be released at colon with alkaline pH, but in the stomach due to acidic pH, this function stops. Thereby, it appears that EU S100 is capable of carrying the probiotic bacteria in the same way as medicines through a safe pathway [16-18]. In order to achieve an appropriate potent in the beads, calcium alginate should be double-coated which firstly would be covered by Chitosan and subsequently by nanoparticles of EU S100 containing probiotic bacteria. This method is absolutely effective as providing the smoothest surface along with high proficiency. Yogurt is one of the most popular fermented dairy products among Iranian over hundred years, which not only is a favorite drink due to its flavors but also is a salient source of probiotics. The viability of these microorganisms relies on some environmental factors, mainly the ultimate pH that is far significant in the growth and stability of probiotics [19-21]. The main purpose of this research is to investigate the impact of calcium alginate Chitosan as well as EU S100 nanoparticles microencapsulation on the stability of probiotic strains including Lactobacillus casei and Lactobacillus bulgaricus under simulated GI and during the phase of storage.
2. MATERIALS AND METHODS
2.1. Preparing of Probiotic Strains
The strains of L. casei and L. bulgaricus were achieved through the commercially lyophilized powder of bacteria (TAK GEN-Iran) (0.2%). At the first stages, strains were cultivated to be enriched a in selective media MRS Broth (De Man-Rogosa-Sharpe) for 48h at 37 °C. 1 mL of each sample cultured in MRS Agar pour-plated anaerobically for 48h at 37 °C (Both media, Merck, Germany). The probiotic growth in log phase was accomplished by a centrifuge (14-Jan, SIGMA, Germany) for 15 min at 4500 rpm.
2.2. Preparing of Chitosan Solution
In order to prepare the above-mentioned solution, we employed the low-molecule of Chitosan (Sigma, USA) approximately 4 g, and then mixed with 90 mL distilled water. For making the acidic criteria 0.4 mL HCl was used. As the pH environment should be adjusted between 5.6 and 5.8, thus, 1 mol L-1 KOH applied. Finally, the liquid was filtered by Whatman paper and sterilized in an autoclave for 15 min at 121°C, afterward, the solution was maintained overnight at room temperature [12, 22, 23].
2.3. Preparing of EU S100 Nanoparticles
Regarding the accessibility of EU S100 polymer in the market, it was mandatory to turn to turn this polymer into nanoparticles using Supercritical Antisolvent Technique (SAS) method which performs as a solvent for EU powder (Evonik Pharma Polymers, Darmstadt, Germany). Generally, 5mg/mL of Eudragit S100 solution (Wisetise, DAIHAN Scientific Co., Ltd, Korea) was blended with distilled water containing 15mg/L Tween 80 (Merck Co, Germany) by centrifuge at 24,000 rpm at 37 °C for 12 min. In order to evaluate the size of particles, lasers device was used [17, 23, 24].
2.4. Microencapsulation Operation of Beads
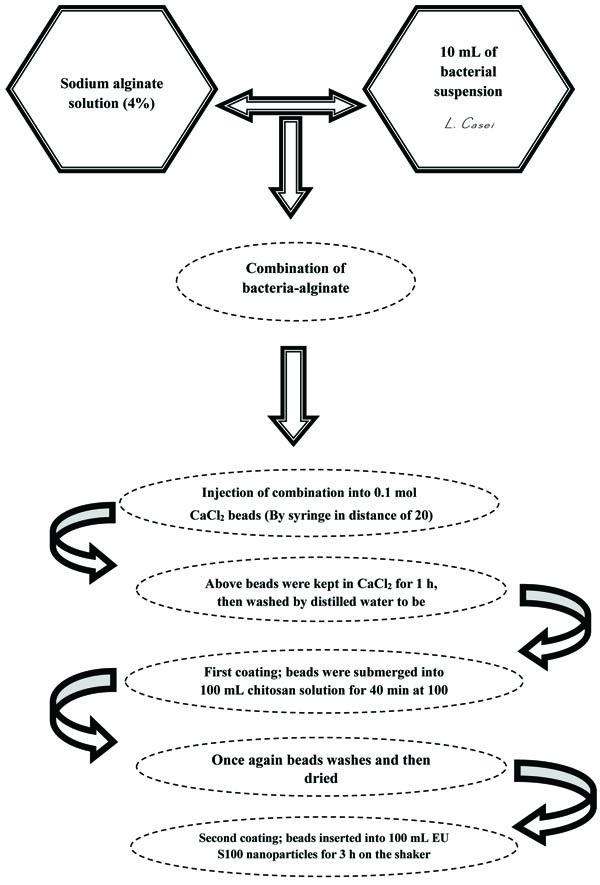
Briefly, 5 g 100 mL-1 sodium alginate (Sigma, USA) mixed with distilled water and maintained at room temperature after sterilization for 12 h. The next stage, 2×1010 CFU mL-1 prepared bacteria suspension added to sodium alginate and dropped by syringe into CaCl2 solution by 15 cm distance. This process leads to forming clot sphere instantly and after a while for 45 min the whole beads were collected and washed by distilled water [17]. For the primary coating of beads, they immersed in 100 mL Chitosan through magnetic stirrer (Domel, SHP10, Slovenian) and then the coated beads washed with distilled water [11, 13, 15]. In the secondary process, single coated beads of the prior stage immersed in 100 mL EU S100 nanoparticle solution. Likewise, the beads inserted in magnetic stirrer for 3 h were the same as the primary coating, they washed [17, 24]. The reason why we applied Chitosan is that it plays a role as a shield layer between the probiotics and environmental conditions to improve the stability of beads. When the Chitosan is vulnerable toward acidic criteria like acidic pH, thereby it must be covered by the second layer to be protected. Nevertheless, the second coat is not as thick, however it performs its duty accurately as a barrier [11, 13, 16, 25].
2.5. Preparation of Probiotic Yogurt
To make a yogurt, we applied 110 g manufactured skim milk (Merck Co, Germany) (10% w/v) in 1 L water and the Erlenmeyer flask was heated for 20 min along with proper blending. After cooling the media in 35 °C the sample inoculated by each strain of L. casei and L. bulgaricus (1 unit 10 L-1) and the pH reached at 4.5 (Isoelectric point) to make clots by cleaving the casein [26]. The inoculated milk was divided into equal portions; one part was added to free probiotic cells (approximately 1010 CFU g-1), whereas the other part was added to beads (1 g beads per 10 g yogurt, 1 g beads containing ~1010 CFU g-1). Finally, all yogurt portions were stored at 4 °C for 40 days.
2.6. Survival Rate of Simulated Microencapsulated and Free Probiotic Bacteria in Gastrointestinal Criteria
We surveyed the viability of probiotics in simulated gastrointestinal conditions during interval time of 0, 8, 16, 24 and 32 days after the inoculation of bacteria in two forms of free and double-coated in yogurts. Generally, 10 g of yogurt contains double-coated and also 10 g of yogurt without beads as a blank sample was inserted in tubes. Furthermore, 0.08 mol L-1 HCl, 2 g L-1 NaCl, and 3 g L-1 pepsin (pH 1.5) in sterile condition were blended in tubes in order to provide GI circumstance. Finally, the tubes were incubated at 37 °C for 30, 60 and 120 min to be assessed. Subsequently, in order to simulate the intestinal conditions for samples, they were transformed to 100 mL of a solution containing 0.05 mol L-1 KH2PO4 and 10 g L-1 bile salt (pH 7.5) and incubated for 3 h at 37°C. For counting, samples diluted by peptone water and separately, 1 mL of each sample was added to MRS agar plates through a pouring-plate method enriched by glucose and samples incubated for 48 h at 37°C, anaerobically. One thing should be considered pivotal is that microencapsulated probiotic bacteria must be released from double-coating layer to be enumerated. Because of this, the beads re-suspended in 90 mL of phosphate buffer (0.1 mol L-1, pH 7.0) were followed by 1 h shaking [27, 28].
2.7. Appraisal of pH and Acidity of Samples and Statistical Analysis
To measure pH and acidity, we applied pH meter device (TES-1380, China) in periods of 0, 8, 16, 24 and 32 days. The viability of two probiotic strains of L. casei and L. bulgaricus were evaluated in a period of 40 days storage using a repeated measure ANOVA test. In addition, the viability of bacteria in GI condition was assessed in periods of 0, 8, 16 and 32 days using repeated measure ANOVA test. Friedman none-parametric test was carried out for comparison acidity and pH in different days. Additionally, mean values of yogurt containing free and coated probiotics as well as the control test on each day was compared using Kruscal-Wallis test. Eventually, the total assessments obtained were in triplicate.
3. RESULTS AND DISCUSSION
3.1. Viability of Strains and Colony Counting
In this study, 100-170 nm sized encapsulated particles were prepared through homogenization of EU S100 powder (24000 rpm, 12 min). After preparation of the EU S100 using SAS technique, the particle size and PDI of EU S100 particles were 120 nm and 0.450, respectively. The ending diameter of the double coated beads was at approximately 70–180 µm. Bacterial count in yogurt containing free and microencapsulated probiotic bacteria is demonstrated in Table 1. Bacterial count carried out twice for each sample and the mean of these repetitions is summarized. To explore the impact of the acidic liquid of the stomach and the bile of the intestine on the viability of microencapsulated probiotic bacteria, an in vitro technique was applied. Bacterial count in simulated GI circumstance is shown in Table 2. The schematic representation of the encapsulation of probiotic bacteria in calcium alginate Chitosan and EU S100 nanoparticles is summarized in Fig. (1).
3.2. Evaluation of pH, and Acidity of Samples up to 40 Days
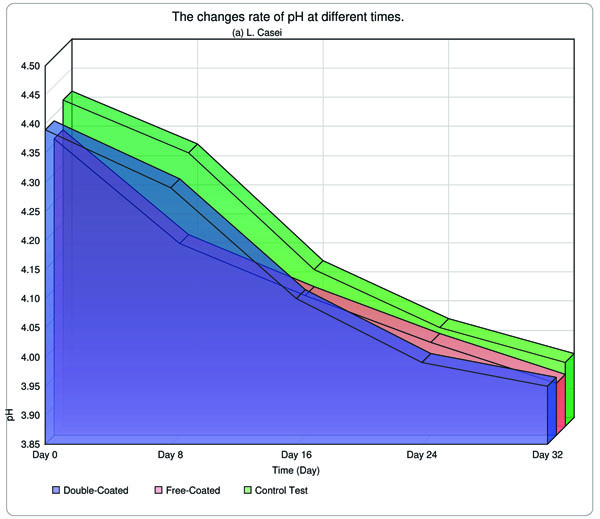
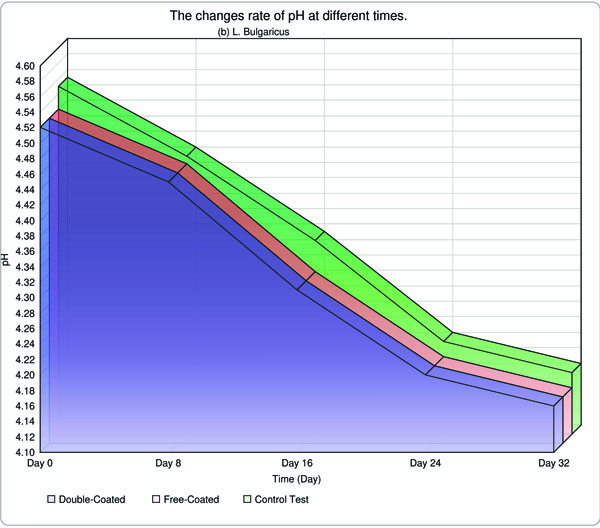
Acidity and pH of yogurt samples were measured on days 0, 8, 16, 24 and 32 days, following incubation and results are indicated in Table 3. Throughout the storage period, the acidity and pH fell and rose, respectively in all samples. Retrospectively, a few studies have been conducted and accomplished worldwide. As a tangible example, Hu et al. (2012) carried out the research in terms of application of EU S100 and acetone by SAS technique to create EU S100 nanoparticle, and they claimed, to achieve access to normal and integrated nanoparticles with 147 nm diameter in 15 MPa at 37°C. It appears that the results were fully appealing. On the contrary, the physico-chemical method such as homogenization was employed to create cleavages in particles, as a result, we were capable of producing a particle with 112 nm, in comparison with the Hu`s investigation, the overall consequence was relatively satisfactory [17].
Regarding Table 1, it is evident that the viability of L. casei which was maintained in yogurt was dramatically diminished and there is an incredible change between the first and last measures. Similarly, L. bulagricus indicated the same behavior, but the rate of decrease was slightly gentle. One interesting point which must be emphasized is that both
| Condition | Strain | Day 0 | Day 8 | Day 16 | Day 24 | Day 32 | Day 40 |
|---|---|---|---|---|---|---|---|
| Free Form | Lactobacillus casei | 6.5×107±9.9×107 | 6.2×106±4.1×106 | 5.7×106±3.7×105 | 5.1×105±3.4×105 | 4.0×105±3.1×104 | 3.8×103±2.7×103 |
| Lactobacillus bulgaricus | 7.3.×109±3.7×109 | 6.9×109±3.3×109 | 8.1×108±4.5×107 | 7.7×107±5.1×106 | 5.2×106±6.1×105 | 6.0×105±2.6×105 | |
| Double | Lactobacillus casei | 7.4×108±3.3×108 | 7.1×108±3.1×108 | 6.5×108±2.2×107 | 5.8×108±1.5×108 | 4.5×108±4.4×108 | 2.8×108±4.0×108 |
| Coated | Lactobacillus bulgaricus | 4.7×109±4.2×109 | 3.1.0×109±3.6×109 | 2.6×109±3.5×109 | 1.5.×109±8.8×108 | 8.0×108±7.6×108 | 7.2×108±6.8×108 |
| Day | Condition | Strain | 0 min | 30 m | 60 m | 120 m |
|---|---|---|---|---|---|---|
| 8 | Free | L. casei | 6.0×106±3.3×103 | 4.4×103±7.7×102 | 6.8×102±0.4×102 | 5.3×101±1.4×100 |
| L. bulgaricus | 7.2×109±3.5×109 | 5.3×107±6.8×106 | 5.5×106±1.9×104 | 6.8×104±2.4×103 | ||
| Double-Coated | L. casei | 6.9×108±1.8×107 | 3.9×107±4.8×106 | 5.9×106±9.2×105 | 3.6×105±7.5×104 | |
| L. bulgaricus | 7.1×108±2.8×107 | 5.6×108±0.0×107 | 9.3×106±2.4×105 | 1.5×106±1.5×104 | ||
| 16 | Free | L. casei | 5.5×106±2.3×104 | 5.9×105±2.1×104 | 6.6×104±1.0×102 | 8.0×102±3.3×101 |
| L. bulgaricus | 8.0×108±0.4×106 | 9.5×105±1.8×105 | 6.2×105±7.8×104 | 3.3×103±2.7×102 | ||
| Double-Coated | L. casei | 6.6×108±1.8×106 | 6.3×107±0.8×106 | 4.2×105±0.1×105 | 3.8×104±9.3×103 | |
| L. bulgaricus | 2.5×108±7.1×107 | 3.5×108±5.1×105 | 7.3×105±0.1×104 | 6.4×105±7.1×103 | ||
| 32 | Free | L. casei | 4.1×105±4.4×103 | 9.1×104±2.5×103 | 6.5×103±2.2×103 | 6.7×101±0.4×101 |
| L. bulgaricus | 5.3×106±0.7×105 | 6.3×105±5.4×103 | 2.4×105±6.6×102 | 4.9×102±5.9×101 | ||
| Double-Coated | L. casei | 4.5×107±5.0×106 | 2.6×106±1.8×105 | 3.0×104±5.8×102 | 2.8×104±1.8×100 | |
| L. bulgaricus | 3.1×107±6.4×103 | 2.7×107±4.4×103 | 4.5×106±5.6×102 | 2.8×104±4.9×102 | ||
| Legend: The scale of calculations is relied on CFU g-1 | ||||||
| Time of Measuring (Day) | Strain | Form of Experiment | pH | Acidity (˚D) |
|---|---|---|---|---|
| 0 | L. casei | Double-Coated | 4.39±0.0 | 97.52±0.0 |
| Free-Coated | 4.36±0.01 | 100.55±0.0 | ||
| Control Test | 4.41±0.0 | 95.51±0.0 | ||
| L. bulgaricus | Double-Coated | 4.52±0.0 | 84.49±0.0 | |
| Free-Coated | 4.50±0.01 | 84.54±0.0 | ||
| Control Test | 4.55±0.01 | 79.52±0.0 | ||
| 8 | L. casei | Double-Coated | 4.29±00 | 105.37±0.0 |
| Free-Coated | 4.18±0.0 | 116.25±0.0 | ||
| Control Test | 4.32±0.1 | 102.20±0.10 | ||
| L. bulgaricus | Double-Coated | 4.45±0.0 | 89.44±0.0 | |
| Free-Coated | 4.46±0.01 | 88.51±0.0 | ||
| Control Test | 4.46±0.1 | 88.52±0.10 | ||
| 16 | L. casei | Double-Coated | 4.10±0.01 | 124.40±0.01 |
| Free-Coated | 4.09±0.01 | 125.41±0.01 | ||
| Control Test | 4.12±0.0 | 122.40±0.0 | ||
| L. bulgaricus | Double-Coated | 4.31±0.0 | 103.38±0.01 | |
| Free-Coated | 4.37±0.1 | 97.35±0.10 | ||
| Control Test | 4.35±0.0 | 99.38±0.0 | ||
| 24 | L. casei | Double-Coated | 3.99±0.0 | 135.35±0.0 |
| Free-Coated | 4.01±0.0 | 133.41±0.0 | ||
| Control Test | 4.02±0.0 | 132.47±0.0 | ||
| L. bulgaricus | Double-Coated | 4.20±0.01 | 114.29±0.10 | |
| Free-Coated | 4.24±0.1 | 110.30±0.10 | ||
| Control Test | 4.22±0.0 | 112.35±0.0 | ||
| 32 | L. casei | Double-Coated | 3.95±0.1 | 139.42±0.10 |
| Free-Coated | 3.94±0.01 | 140.45±0.0 | ||
| Control Test | 3.96±0.1 | 138.51±0.10 | ||
| L. bulgaricus | Double-Coated | 4.16±0.01 | 118.55±0.0 | |
| Free-Coated | 4.20±0.01 | 114.60±0.0 | ||
| Control Test | 4.18±0.0 | 116.64±0.0 |
double-coated probiotic strains performed entirely different rather than the free condition as the viability of strains declined moderately at the final assessment. To illustrate other studies in this area, Krasaekoopt et al. (2003 and 2004) [29, 30] as well as Krasaekoopt, Bhandari, & Deeth, (2004) [31] evaluated separately the only one-coated beads by Chitosan and the viability of Lactobacillus acidophilus 574 L. casei 01 and Bifidobacterium bifidum 1994, was checked in yogurt during the storage of dairy product. In the two above-mentioned studies, the results revealed that the viability of the coated strains by Chitosan is far higher than the conventional form with the vicinity of 1 log. The number of Lactobacilli strains was higher than 107 CFU g-1, conversely for Bifidobacterium strains during the storage. Earlier, Chávarri et al. (2010) [11], Hansen, Allan-Wojtas, Jin, & Paulson, (2002) [32], Kanmani et al. (2011) [13, 21, 26] and Krasaekoopt & Watcharapoka (2014) [31, 32] conducted an investigation about the viability of free and encapsulated probiotic bacteria under GI circumstance where everyone achieved comparative findings.
Since we employed doubled-coated methods, hence, we expected that the rate of stability of bacteria in GI condition would be higher than the only one layer coating in the storage phase because earlier it was mentioned that the second layer acts as a barrier. Table 2 reflects another aspect of the viability of bacteria under gastrointestinal stress. In this study, both L. bulagricus and L. casei showed acceptable stability to extreme conditions when they were counted in marked days, which has exclusively been observed in this research. On a counting stage, the number of bacteria was different as the number of L. bulagricus was more than others. In comparison with previous studies, this is the first time that the viability of strains covered with beads has been evaluated in the yogurt as a dairy product. After counting, the beads were extracted from yogurt in order to be transferred to an environment with GI features (Fig. 2). The number of free cells of L. casei and L. bulgaricus decreased from 6.0×106 and 7.2×106 on day 0 to 4.1×105 and 5.3×106 in day 32, respectively in GI condition. Moreover, double-coated L. casei and L. bulgaricus declined from 6.9×108 and 7.1×108 to 4.5×107 and 3.1×107 in simulated condition.
The yogurt samples containing the free form of bacteria, acidity and pH alterations were found to be substantial than those encapsulated. Overall, in this research, the pH and acidity of both L. bulagricus and L. casei in yogurt were evaluated separately, thus appealing results were obtained which are reported in Table 3. It can be surmised that as the time went by, pH was steadily decreased, however, these rates were found to be most dramatic in the first days and gradually became more moderate in the two last measurements (16th and 32nd days). This trend was valid for both strains and by analyzing the data, astonishing results were obtained, in which the rates of pH and acidity in both double-coated, as well as typical forms, reached far close to the control test in the final measurement (32nd day) for both strains, especially in L. casei strain (Fig. 3).
CONCLUSION
The main purpose of this investigation was to clear the impact of microencapsulation (Double-Coated) on strains viability in both lumen and food product, like yogurt, and make a comparison with cells in normal criteria. This study confirms that the results obtained in previous researches by others, by employing microencapsulation for lactic acid bacteria mainly L. casei and L. bulgaricus, act as an extender of viability. Consequently, these bacteria live longer than in the usual circumstance. In particular, the impacts of microencapsulation were assessed in two conditions of simulated GI stress and their presence in fermented dairy product namely yogurt. This study showed that microencapsulation on bacteria, considerably increases the viability in both normal and simulated conditions. Likewise, the applied method would be able to descend the metabolic interactions of bacteria, leading to an appropriate level of pH during the maintenance phase of the ultimate product. The pattern of pH changes was similar for both strains and revealed that the rates of pH and acidity in both double-coated and normal forms are close to control the test in the final measurement.
Other relevant studies had proven that the coherence of pH and acidity incredibly plays an irrefutable role in improving some organoleptic attributes like texture, flavor, and aroma. Exclusively, the second coating of EU S100 was taken as a shield for the primary layer which causes the LAB to have the maximum number and function in foodstuffs, stomach, and colon in human health. Finally, as a suggestion, it demands more consideration by researchers to evaluate the role of encapsulation in other food products, such as fruit juices and biscuits, and also increase the coating stability of beneficial bacteria against the immunity system.
LIST OF ABBREVIATIONS
| GI | = Gastrointestinal |
| EU | = Eudragit |
| LAB | = Lactic Acid Bacteria |
| SAS | = Supercritical Antisolvent Technique |
| PDI | = Polydispersity Index |
| CFU | = Colony Forming Units |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for the study that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author wishes to appreciate Dr. Chang for his guidance in this research. However, the financial expenses are fully covered by the author.