RESEARCH ARTICLE
Biosynthesis and Characterization of Silver Nanoparticles Using Phoenix dactylifera Fruits Extract and their In Vitro Antimicrobial and Cytotoxic Effects
Sarvat Zafar1, *, Aiman Zafar2
Article Information
Identifiers and Pagination:
Year: 2019Volume: 13
First Page: 37
Last Page: 46
Publisher ID: TOBIOTJ-13-37
DOI: 10.2174/1874070701913010037
Article History:
Received Date: 09/01/2019Revision Received Date: 14/03/2019
Acceptance Date: 15/03/2019
Electronic publication date: 30/04/2019
Collection year: 2019

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
In this article, silver nanoparticles (AgNPs) were synthesized by the biological green technique, using the aqueous extracts obtained from fruits of Phoenix dactylifera (date palm). This method is simple, rapid, non-toxic, and sustainable, and substitutes for the conventional physical/chemical methods. The cytotoxic activities of AgNPs derived from date fruit extract have not been mentioned in the earlier studies.
Methods:
The biosynthesized AgNPs are analyzed by Fourier transform infrared spectroscopy (FT-IR), UV-visible spectroscopy (UV-vis), and Transmission Electron Microscopy (TEM) methods. The assessment of antimicrobial effect towards human pathogenic microbial strains and their potential cytotoxicity against human breast cancer cell lines (MCF-7) were also evaluated.
Results:
FT-IR spectral studies showed that phytomolecules such as carbohydrates, phenolic acids and flavonoids present in date fruits extract are involved in the reduction and capping of the AgNPs. UV-vis spectrum revealed Surface Plasmon Resonance (SPR) at 425 nm which attributes the presence of AgNPs in aqueous extract. TEM micrographs showed that AgNPs particle diameter is ranged from 20 nm to 100 nm with spherical morphology. The biosynthesized AgNPs exhibited significant antimicrobial activity towards human microbial strains. Phytosynthesized NPs also induce cytotoxicity via necrosis, apoptosis and mitodepressive mechanisms that can disturb the cellular components at various stages of cell cycle.
Conclusion:
The present study concludes that biologically synthesized AgNPs using Phoenix dactylifera is cost-effective, rapid, non-toxic, and sustainable and can be effectively used as an adjunct for the treatment of breast carcinoma.
1. INTRODUCTION
Plant sources have been considered as one of the most essential resources for bio-green approaches focused on the formation of nanoparticles (NPs) [1-10]. Numerous strategic approaches have been employed for the biosynthesis of NPs from metal ions of the analogous metals [11-13]. Among this, biological synthesis is advantageous not only because of eco-friendlier approaches compared with some of the physicochemical strategies but however, it can also produce a huge amount of NPs [14-18]. Therefore, biological methods employing aqueous extracts derived from plant materials have emerged as a simple and single step protocol and a viable substitute for chemical and physical synthetic procedures [19-24]. Due to the immense potential of phytochemical synthesis of AgNPs, its application and development are ever growing in the area of advanced technologies [25-27]. AgNPs are found to be widely used in various fields such as antibacterial, antiviral, anti-inflammatory, catalytic, sensing and optical properties [28-31]. Several works on the preparation of Ag nanoparticles utilizing plant-mediated extract materials have also been used such as Medicago sativa [32], Aloe barbadensis [33], Emblica officinalis [34], Hibiscus rosasinensis [12], Capsicum annum [35], Momordica charanga [36], Olea europaea [37], Mangifera indica [38] and Citrus sinensis [39].
Date fruit (Phoenix dactylifera) is a tropical and subtropical tree and belongs to family Arecaceae (40). It is widely found in Saudi Arabia, Iran, Iraq, Egypt, UAE, Algeria, Sudan, Oman, and Pakistan for its edible sweet fruit. The Phoenix dactylifera is regarded as an inexpensive fruit and rich in phytomolecules and antioxidants. The major biomolecules extracted from date fruit are carbohydrates, phenolics, sterols, carotenoids, anthocyanins, and flavonoids [41]. The fruit-mediated AgNPs obtained from the bioreduction of date extract have been reported [42, 43]. However, cytotoxic activities of AgNPs in human cancer cell lines (MCF-7) by using date palm fruits extract are not reported in earlier studies. Moreover, the rate of bioreduction by using date fruit extract is extremely fast and produced more stable nanoparticles as compared to NPs derived from other plant resources, microbes and seaweeds [42]. This study is cost-effective because it does not require any additional solvents, emulsifiers as well as consumed less energy for the synthesis of NPs. Moreover, Phoenix dactylifera is cheap and widely available fruit in Saudi Arabia. The above-mentioned procedure only requires the purification of nanoparticles through centrifugation method. Hence, the overall cost for biologically synthesized AgNPs is reduced to 60% compared to the synthetic drugs used for the treatment of breast carcinoma.
The objective of this article is to report one-step biosynthesis of AgNPs using aqueous fruit extract from Phoenix dactylifera. Generation of silver NPs are analyzed by using UV-visible and FT-IR spectroscopy, and particle size was confirmed through TEM analysis. The assessment of antimicrobial effect towards human pathogenic microbial strains and their potential cytotoxicity assay in MCF-7 cell lines were also evaluated.
2. MATERIALS AND METHODS
Silver nitrate (AgNO3) of analytical grade was purchased from Sigma-Aldrich Chemicals and was used without any further purification. Fresh Phoenix dactylifera (Fig. 1) fruits were purchased from local market in Jazan, Saudi Arabia. All of the solutions and chemical were prepared in the de-ionized water.
2.1. Preparation of Aqueous Phoenix dactylifera Fruit Extract
The fruits of Phoenix dactylifera were repeatedly washed with de-ionized water to remove dust and soil particles and were also surface sterilized by using 0.1% mercurous chloride (HgCl2) for 1 min to remove microbial stains [44]. 20 gm surface sterilized fruits were cut into small pieces and add 100 ml of double distilled-ionized water in it and stirred for 30 mins at 60oC. The aqueous fruit extract was cooled to ambient temperature and filtered twice by using Whatman No.1 filter paper. The obtained light yellow extract was collected as a stock solution stored at 40C in the refrigerator used for further studies.
2.2. Preparation of AgNPs
1mM AgNO3 solution was prepared in the de-ionized water. AgNPs were biosynthesized by mixing 3 ml of aqueous Phoenix dactylifera fruit extract with 9 ml of 1mM AgNO3 solution. Reaction products were heated for 20 mins at 60°C under constant stirring. The color of the aqueous mixture was changed from yellow to brown. This is due to the bioreduction in the aqueous solution, which indicates the formation of AgNPs and was identified through UV-vis spectra. The similar procedure was adopted for biosynthesis of AgNPs at different time intervals (5, 10 and 15 mins). The obtained AgNPs solution was centrifuged at 3000 rpm for 20 mins and the final product was redispersed in 10ml distilled water. The procedure of centrifugation and redispersion was done twice. AgNPs were then dried in an oven at 60°C for one hour. Finally, the obtained product was ground into powder for further studies.
 |
Fig. (1). Phoenix dactylifera fruits. |
3. CHARACTERIZATIONS
3.1. UV-visible Spectroscopy (UV)
UV-vis absorption spectrophotometry was used to analyze the optical properties of AgNPs. The UV-visible absorption spectra of aqueous AgNPs were recorded on a double beam UV-visible spectrometer (6800, JENWAY) with a resolution of 2 nm and path length of 1 cm in 300–600 nm range.
3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR spectra were recorded in FT-IR spectroscopy with wavelength region from 4000 cm-1 to 400 cm-1 at a resolution of 4cm-1. Infrared spectra were obtained using a Thermo Scientific Nicolet iS10 FT-IR spectrophotometer [32].
3.3. Transmission Electron Microscope (TEM)
The particle size and morphology of biosynthesized nanoparticles were analyzed by using Morgagni 268-D TEM, FEI, USA operated at an accelerating voltage between 80 and 200 kV. For TEM analysis, samples of AgNPs were prepared by placing a small volume of the aqueous solution onto the carbon coated copper TEM grids and vacuum dried.
3.4. In Vitro Antimicrobial Studies
The antimicrobial studies of the biosynthesized silver NPs were assessed using strains against four different pathogenic bacteria namely Escherichia coli (MTCC 443), Pseudomonas aeruginosa (MTCC 424), Staphylococcus aureus (MTCC 96), Enterococcus faecalis (MTCC 439) and one yeast fungi Candida albicans (MTCC 227) by well diffusion methods [45]. Mueller Hinton Agar (MHA) obtained from Himedia (Mumbai) was used in In vitro antimicrobial studies. The MHA plates were prepared by using 25 ml of molten media into sterile Petri plates. These petri plates were then solidified and grown about 18 h (OD adjusted to 0.6). 100 µl of microbial strains cultures were placed onto a plate and made culture lawn. After five minutes, a sterile cork borer was used to make wells of 6 mm in size on the agar puncture. Different concentrations of aqueous silver nanoparticles (20, 40, 60, 80, 100 lg/ml) are placed into the agar well with positive control Streptomycin for bacterial strains and Clotrimazole for fungal strains. The petri dishes were kept for the incubation for 24 hours at 370C, and the inhibitory zone was measured in millimeter.
3.5. Cytotoxicity Assay (MTT)
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) method was used to study the cytotoxicity assays of silver nanoparticles obtained from date fruit extract on MCF-7 cell lines [46]. In the colorimetric method, MTT is reduced to purple formazan by mitochondrial Succinate dehydrogenase. MCF-7 cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum solution. The cells were seeded with 24 well culture plate along with different concentrations of AgNPs (12.5, 25, 50, 75, and 100, 125, 150, 175, and 200 µg/ml) and incubated at 37°C in a humidified CO2 incubator with 5% CO2. After 48 h incubation, 100µl MTT was poured into each well and incubated for 3h in room temperature until a purple product formazan was formed. The obtained formazan crystals were dissolved by using 100μl SDS in DMSO and absorbance was measured in Lark LIPR-9608 microplate reader at 540 nm wavelength. Absorbance for the untreated cells absorbance is considered as a control reference. The percentage of cell viability was calculated as follows [47, 48]
Percentage of cell viability = OD sample / OD control X 100
4. RESULTS AND DISCUSSION
4.1. Visual and UV-Visible Absorption Studies
Fig. (2) shows the visual appearance of date palm fruit-mediated AgNPs. The appearance of color change from yellow to brown during reaction attributes the generation of silver nanoparticles. The aqueous date extracts rich in polyphenols, antioxidants, and flavonoids. The hydroxyl groups present in biomolecules are responsible for the bioreduction of Ag+ ions as shown in the mechanism (Fig. 3)
The phyto-reduction of silver ions to AgNPs was analyzed by UV-vis spectroscopy. Fig. (4) shows the spectra of AgNPs prepared at different time intervals (5-20 minutes). As seen in the spectra, the intensity of absorbance bands increases as the reaction time of the reacting mixtures was increased. The maximum absorption appeared in the visible range at 425 nm after 20 minutes. This is due to SPR in silver NPs solution, which attributes the formation of silver nanoparticles. This showed that Ag+ ions were completely reduced to nanoparticles within 20 minutes in the presence of date fruit extract. Several researchers reported the SPR bands of AgNPs in the wavelength of 425-460 nm [49-52].
4.2. FT-IR Studies
FT-IR spectroscopy analysis was used to identify the functional groups of the biomolecules present in the fruit extract. As reported in the previous studies, date fruit is rich in phytomolecules such as carbohydrates, phenolic acids, sterols, carotenoids, anthocyanins, procyanidins and flavonoids [41]. The FT-IR spectrum of date extract Fig. (5A), shows characteristic stretching bands for phenolic O–H, C=O, and C–OH at 3450, 1639, and 1011 cm–1, respectively. Absorption bands for C–H stretching and –OH bend for polyphenols also appeared at 2918 and 1420 cm-1 [53] that confirms the presence of aromatic compounds like flavonoids and terpenoids in fruit extract, which consist of many C-H bonds. The band at 1011 cm-1 were attributed to C-O stretching and secondary –OH which depicts the presence of phytomolecules in the aqueous date fruit extract [54].
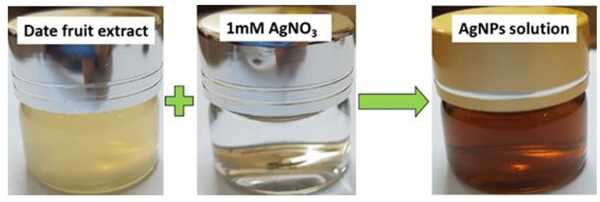 |
Fig. (2). Visual appearance of AgNPs after addition of date fruit extract to AgNO3 solution. |
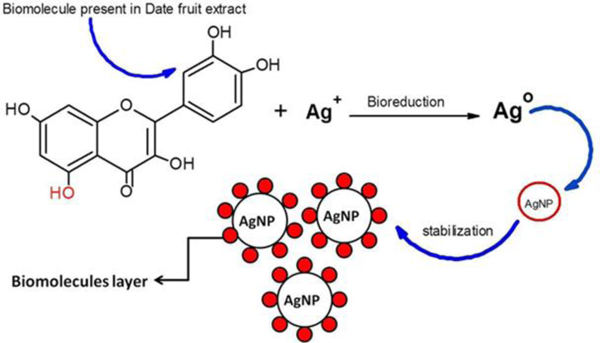 |
Fig. (3). Bioreduction and stability of AgNPs using date fruit extract. |
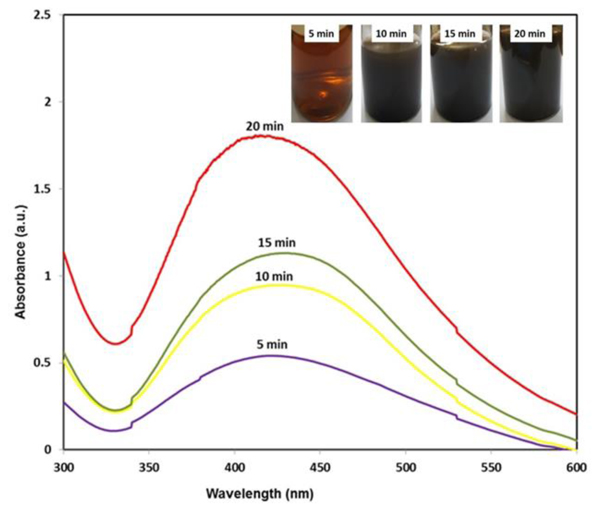 |
Fig. (4). UV-Visible spectra of AgNPs at different reaction time. |
In the spectrum of AgNPs (Fig. 5B), a strength of bands decreases at 3450, 1639, 1420 and 1011 cm–1 that clearly indicates the adsorption of natural polyphenols on the surface of the AgNPs. The band at 550 cm-1 for AgNPs is also observed in Fig. (5B), which corresponds to the bioreduction of Ag+ ions [55].
4.3. TEM Analysis
The particle size and morphology of the phytosynthesized silver nanoparticles obtained from date extract were determined using TEM. From the TEM micrographs, it was depicted that AgNPs particle diameter is ranged from 20 nm to 100 nm with spherical morphology. As can be seen in Fig. (6), the morphology of the NPs is almost homogeneous and some are in aggregated form. Nanomaterials have very high surface energy and small particle diameter, they can be easily aggregated. The average particle diameter of the nanoparticles is found to be correlative to the range of AgNPs derived from fruit extracts of H. undatus, S. nigra, and M. domestica [19-21].
4.4. In Vitro Antimicrobial Studies of AgNPs
Biosynthesized silver NPs were investigated for their potential antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus faecalis and one yeast fungi Candida albicans by agar well diffusion method. As seen in Table (1), the diameters of inhibition zones showed varied responses against all the tested human pathogenic strains. It has been analyzed that antimicrobial studies were in a dose-dependent manner [56, 57]. Silver nanoparticles phytosynthesized using date palm extract revealed highest inhibition zone (34.0 mm) at a concentration (100 µl) against C. albicans and followed by E. coli (30.0 mm), P. aeruginosa (29.0 mm), E. faecalis (27.0 mm) and S. aureus (29.0 mm), respectively. The result showed low inhibitory activity against E. faecalis (22.0 mm) at a concentration (25 µl) (Fig. 7). It was shown that the inhibition region is increased with increase in the concentration of nanoparticles against all the tested microbial strains.
Silver nanoparticles were found to be non-toxic in human cells and showed significant growth inhibitory activities on microbial pathogens because of their small dimensions and greater surface area [58, 59]. Biosynthesized nanoparticles attached to the microbial cell membrane causing plasmolysis and finally inhibit the membrane synthesis [60, 61]. Silver NPs interact with functional moieties of the microbial cell such as thiol, hydroxyl, and a carboxyl group and disturbs the respiratory protease enzyme [62-64]. Furthermore, nanoparticles entered into bacterial cells and release silver ions that inhibit DNA replication, cell division, and finally leads to the death of bacterial cell [65, 66]. However, the mechanism of nanoparticles with various human pathogens are not yet completely known.
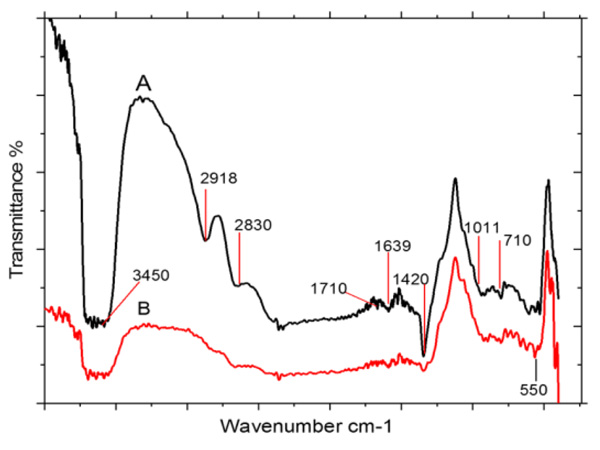 |
Fig. (5). FT-IR spectra of (A) date palm aqueous fruit extract and (B) biosynthesized silver nanoparticles. |
| Concentration of AgNPs, Diameter of Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|
| Test Strains | 25 µl/well | 50 µl/well | 75 µl/well | 100 µl/well |
ZOI(mm) Positive Control 30 µg/well |
| Escherichia coli | 24 | 26 | 28 | 30 | 16* |
| Pseudomonas aeruginosa | 26 | 27 | 28 | 29 | 18* |
| Staphylococcus aureus | 24 | 24 | 24 | 25 | 26* |
| Enterococcus faecalis | 22 | 23 | 24 | 27 | 20* |
| Candida albicans | 26 | 28 | 30 | 34 | 22 |
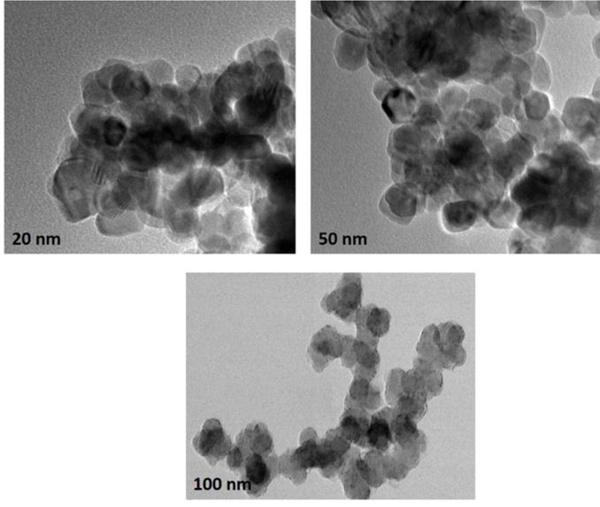 |
Fig. (6). TEM micrographs of Silver nanoparticles obtained using date fruit extract. |
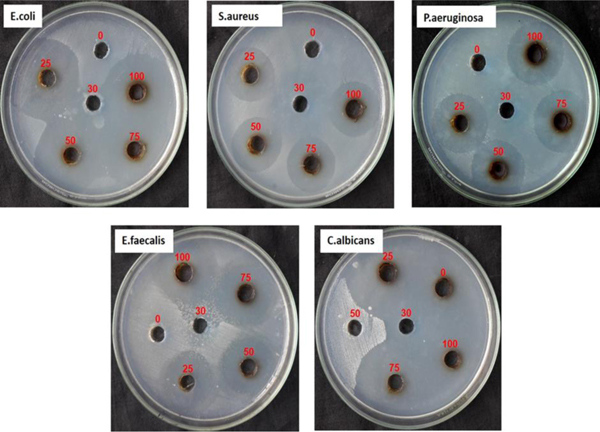 |
Fig. (7). Antimicrobial activity of AgNPs against different microbial pathogen. |
4.5. Cytotoxicity Assay
Cytotoxic activities of biosynthesized AgNPs were studied on MCF-7 breast cancer cell lines using the MTT method. MTT data showed cytotoxicity of NPs towards MCF-7 cell lines in dose-dependent manner (12.5, 25, 50, 75, 100, 125, 150, 175, and 200 µg/ml). Significant cytotoxic activity was observed in biosynthesized nanoparticles with IC50 90.8 µg/ml. Nanoparticles have the ability to kill 50% of the cells and this indicates the effects of cytotoxicity. As can be seen in Fig. (8), the cell viability decreases with increase in the concentration of silver NPs. The highest inhibitory effect was observed at a concentration of 200µg/ml of AgNPs (Figs. 8 and 9). Cytotoxicity of fruit-mediated AgNPs against MCF-7 cell lines is also reported in different plant species [19, 67-69]. Several mechanisms have been mentioned in the previous studies that nanoparticles induce cytotoxicity via Reactive Oxygen Species (ROS) that can damage all cellular moieties and finally lead to cell death through the necrosis [70]. This also increases the intracellular oxidative stress that results in cell death by the process of apoptosis [71]. Silver NPs have anti-angiogenic properties that have the ability to block the activity of abnormally expressing signal proteins and prevent the formation of new fine capillaries that are needed for tumor growth and metastasize [72]. The present study showed the mitodepressive and cytotoxic effect of nanoparticles. This is achieved by DNA inhibition at S-phase. Induction of AgNPs increases mitotic abnormalities which disturb both metaphase and anaphase. Finally, this effects both the impairment of mitotic spindles and disturbs the orientation of chromosomes at various stages of the cell cycle [73]. Chromosome stickiness is also induced by AgNPs [74, 75]. The induction of chromosomal breaks and micronuclei shows the clastogenic potential that results in loss of genetic material [76]. Thus, the above-mentioned factors suggest that AgNPs possess mitodepressive, mitoclassic and clastogenic properties and can be effectively used as an adjunct for the treatment of breast carcinoma.
 |
Fig. (8). Cytotoxicity of AgNPs towards MCF-7 cell lines. |
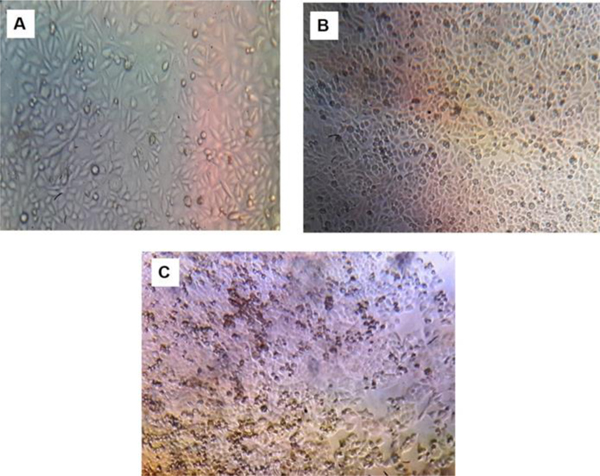 |
Fig. (9). Morphology of control (A), biosynthesized AgNPs 100 µg/ml (B) and 200 µg/ml against MCF-7 cell lines. |
CONCLUSION
AgNPs were prepared from aqueous fruit extract of Phoenix dactylifera and AgNO3 solution via. biological method. Phytoreduction of silver ions was analyzed by UV-visible spectra that showed a broad absorption band at 425 nm. Phytomolecules present in aqueous fruit extract responsible for bioreduction and capping of nanoparticles was evident from FT-IR studies. Particle size was analyzed by TEM which is ranged from 20 -100 nm with spherical morphology. The biosynthesized AgNPs exhibited significant antimicrobial activity towards human microbial strains. Phytosynthesized NPs also induce cytotoxicity via necrosis, apoptosis and mitodepressive mechanisms that can disturb the cellular components at various stages of the cell cycle. The present study concludes that biologically synthesized AgNPs using Phoenix dactylifera are cost-effective, rapid, non-toxic, and sustainable and can be effectively used as an adjunct for the treatment of breast carcinoma.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for these studies.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.








