All published articles of this journal are available on ScienceDirect.
Use of Aqueous Two-Phase and Three-Phase Partitioning Systems for Purification of Lipase Obtained in Solid-State Fermentation by Rhizopus arrhizus
Abstract
Background:
Purification of enzymes by conventional methods such as precipitation and chromatographic techniques is a costly and time-consuming procedure and may lead to low yields of enzyme activity. Alternative liquid-liquid extraction methods such as Aqueous Two-Phase Systems (ATPS) and Three Phase Partitioning (TPP) are characterized by the high enzyme yields and purification degree.
Objective:
The objective of this study was the application of partitioning systems ATPS and TPP for purification of lipase produced in solid-state fermentation by Rhizopus arrhizus.
Methods:
ATPS and TPP were used for purification of lipase, obtained by solid state cultivation of Rhizopus arrhizus.
Results:
Lipase was isolated with PEG4000/potassium sodium tartrate ATPS and the effect of the system composition, including PEG 4000 and potassium sodium tartrate concentrations on lipase partitioning was studied. When using 30% PEG4000/21% potassium sodium tartrate, lipase was distributed in the top phase, and the highest recovery yield of 217% and purification fold of 6.1 were achieved. It was found that at PEG4000 concentration of or higher than 15%, the enzyme was present in the top polymer-rich phase with a partitioning yield of over 90%. Upon application of TPP for lipase isolation, the effect of t-butanol concentration, ammonium sulfate concentration and pH on enzyme partitioning was investigated. The highest lipase recovery yield of 71% and 19.1-fold purification were achieved in the interfacial phase in the presence of 30% ammonium sulfate saturation with 1.0:0.5 crude extract/t-butanol ratio at pH 7 in a single step. The sodium dodecyl sulfate-polyacrylamide gel electrophoresis and zymographic analysis showed significant purification of lipase by TPP and the presence of two multiple forms of the enzyme.
Conclusion:
ATPS (PEG4000/ Potassium sodium tartrate) and TPP (1.0:0.5 crude extract/t-butanol ratio, 30% ammonium sulfate saturation, pH 7) proved to be rapid methods for the isolation and purification of lipase and they can be used in downstream processing for industrial preparation of the enzyme.
1. INTRODUCTION
Isolation and purification of biomolecules are two of the critical stages determining the efficiency of biotechnological processes. Purification step itself makes up more than 70% of the total processing costs [1]. Conventional methods for isolation and purification of biomolecules include several consequent steps, which are usually costly procedures, and through them, significant loss of the target product may be achieved.
Enzymes are protein structured molecules that are widely used as catalysts for chemical reactions in various industry branches. The production of enzymes is one of the most intensely developing areas in biotech industry. Often enzymes may be purified several hundred-fold, but the yield of activity may be very low, frequently below 10%. However, some enzyme applications require their purification. It is, therefore, necessary to perform research on enzymes’ purification procedure, which is strictly specific for each individual enzyme [2].
Various conventional purification methods are applied for enzymes’ isolation. They include ammonium sulfate precipitation followed by size-exclusion and ion exchange chromatography, hydrophobic interaction chromatography, affinity chromatography, or some combination of these methods [3]. The use of chromatographic techniques in some cases may lead to low yields of enzyme activity, as was observed for the process of lipase purification. Dandavate et al. isolated lipase from Burkholderia multivorans V2 using the initial treatment of the culture fluid with CaCl2, acetone precipitation and subsequent size exclusion chromatography with Sephadex G150 at a final yield of 0.96% [4]. Bhosale et al. isolated lipase from Bacillus sonorensis 4R yielding 1.98% by sequential ammonium sulfate precipitation and DEAE-cellulose ion exchange chromatography [5].
Alternative to conventional methods for enzymes isolation and purification are liquid-liquid extraction methods such as an Aqueous Two-Phase System (ATPS) and Three Phase Partitioning (TPP). The implementation of these methods does not require the availability of expensive equipment or reagents. Protein purification by ATPS is a promising method, characterized by high yield and high purification degree. The system consists of two liquid phases, which are not intermixed above a certain critical concentration. ATPS with practical application include polymer/salt (PEG/K2HPO4, PEG/(NH4)2SO4), polymer/ polymer (PEG/dextran) or alcohol/salt (1-propanol/(NH4)2SO4) [6].
ATPS consisting of polymer/salt (PEG/K2HPO4, PEG/ (NH4)2SO4) were used to isolate and purify microbial lipases. An effective ATPS for lipase purification was 20% PEG 8000/18% K2HPO4 and 6% NaCl at pH 6.0 and 4°C; a purification degree of 201.53-folds was achieved [7]. Zhang and Liu [8] used ATPS consisting of 12% PEG 4000/13% K2HPO4 and 2% NaCl at pH 7.0 to partially purify lipase from Trichosporon laibacchii. A purification rate of 5.84 folds and an enzyme yield of 80.4% were obtained [8]. Anvari [9] extracted lipase from Rhizopus microsporus during fermentation process by ATPS of 20% PEG 2000/12% (NH4)2SO4 and 5% Na2CO3 at pH 8.0. The yield was 92.3% and the purification degree was 19.8 folds [9].
Another method suitable for isolation and purification of biomolecules is TPP. The technique reduces separation time, enables working at room temperature and allows recycling of chemicals [3].
The method involves the addition of a salt to the aqueous suspension, followed by the addition of an organic solvent [10]. Ammonium sulfate is most commonly used as a salt component, and t-butanol is used as an organic solvent. After adding critical concentrations of ammonium sulfate and tbutanol, the proteins found in the dissolved state are separated into the third phase (interphase) formed at the boundary between the bottom aqueous phase and the top t-butanol phase [11].
Kuepethkaew et al. established the highest recovery yields of 87.41% lipase activity using t-butanol in the formation of TPP, whereas with 1-propanol, 2-propanol, and 1-butanol, the highest yields of lipase activity varied around 50% [10]. The authors used consequently TPP with crude extract/t-butanol ratio of 1.0:1.0 in the presence of 50% (w/v) (NH4)2SO4 and ATPS containing 25% PEG1000/15% MgSO4 at pH 5.0 for lipase purification from the white shrimp (Litopenaeus vannamei). As a result of the sequentially applied techniques, they obtained 5.19 purification fold with 78.46% yield [10].
The objective of this study was to demonstrate the use of the partitioning systems ATPS and TPP for purification of lipase produced in solid-state fermentation by Rhizopus arrhizus.
2. MATERIALS AND METHODS
2.1. Preparation of Lipase
The lipase tested was obtained in solid-state cultivation of Rhizopus arrhizus. The composition of the nutrient medium, the cultivation conditions and the procedure for enzyme extraction were described in previous studies [12].
2.2. Phase Diagrams and ATPS Preparation
The binodal curves were estimated using turbidometric titration. All ATPS were prepared in 15 mL graduated centrifuge tubes. To study the effects of PEG and salt concentration on the partitioning of lipase from the enzyme extract, different concentrations of PEG 4000 and potassium sodium tartrate were mixed. The tubes were shaken on a vortex for 2 min, followed by centrifugation for 20 min at 4000 rpm to assist phase separation. After phase separation and visual estimation of the top and bottom phases, the volumes of the phases were used to estimate the volume ratio. The samples of the top and bottom phases were carefully withdrawn and lipase activity and protein concentration were determined in each phase [13].
2.3. Determination of Selectivity, Purification Fold and Yield
The partition coefficient for lipase activity (Ke) in the ATPS was calculated:
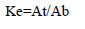 |
(1) |
where At and Ab are the enzyme activity in the top and bottom phases (U/ml).
The protein partition coefficient (Kp) in the ATPS was defined as:
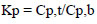 |
(2) |
where Cp,t and Cp,b are the concentrations of protein in the top and bottom phases (mg/ml).
Partitioning yield of lipase in the top phase (PYL, %) was also calculated:
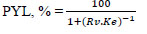 |
(3) |
where Rv is the volume ratio of the top phase to the bottom phase (Vt/Vb).
Selectivity (S) was calculated as:
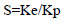 |
(4) |
The purification fold of lipase in the top phase (PFtop) was defined as the ratio of the specific activities in the top phase and of the crude enzyme (U/mg). The specific activity is the ratio of total lipase activity (U) to the total protein (mg).
The recovery yield of lipase was defined as:
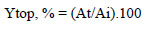 |
(5) |
where At is the total lipase activity in the top phase and Ai is the total initial lipase activity (U).
2.4. TPP Preparation
TPP system was formed with a simultaneous addition of salt directly to the crude enzyme extract followed by an organic solvent addition [10]. TPP was carried out as described by Duman and Kaya [11] with modifications.
Purification of lipase by TPP was monitored by determination of the purification fold and the recovery yield. The purification fold was defined as the ratio of the specific activities (U/mg) of the purified lipase and the crude enzyme. The recovery yield of lipase was defined as the ratio of the total activity of the purified lipase (U) to the total initial lipase activity (U) of the crude enzyme.
2.5. Effect of t-Butanol on Partitioning of Lipase by TPP
The extract, containing lipase (3 ml) was mixed with 30% (w/v) ammonium sulfate at 25°C, vortexed for 2 min and different ratios (v/v) 1.0:0.5, 1.0:1.0, 1.0:1.5, 1.0:2.0 of t-butanol were added. The mixture was incubated at 25°C for 1 h and centrifuged at 6000 rpm for 5 min for the formation of the three phases. The top organic phase, the middle interfacial precipitate, and the bottom aqueous phase were separated carefully, and after the dissolution of the interfacial precipitate in water, they were assayed for lipase activity and protein content.
2.6. Effect of Ammonium Sulfate Saturation on Partitioning of Lipase by TPP
The effect of saturation with ammonium sulfate at concentrations of 20, 30, 40, and 50% was investigated at a constant crude extract/t-butanol ratio of 1.0:0.5.
2.7. Effect of pH on Partitioning of Lipase
The effect of pH on partitioning of lipase by TPP was studied at pH values 6, 7, 8, and 9, at 30% ammonium sulfate saturation and crude extract/t-butanol ratio 1.0:0.5 [11].
All experiments on lipase partitioning were performed in triplicate, and the differences in the readings were less than ±10%. The experimental results were expressed as mean values ± standard errors.
2.8. Determination of Lipase Activity
For lipase activity determination, the method proposed by Babu et al. [14] and Saifuddin et al. [15] was adapted. The substrate solution was prepared by dissolving 30 mg ρ-nitrophenyl palmitate in 10 ml isopropanol, mixed with 90 ml 0.05 M Tris-HCl buffer with pH 7.2, 0.4 g Triton X-100 and 0.1 g gum Arabic. 2.4 ml of the substrate solution was incubated at 35°С for 10 min and 0.1ml suitably dissolved enzyme was added. The enzyme reaction was performed at 35°С for 30 min and the enzyme was inactivated by the addition of 1.0 ml 0.5 M solution of EDTA with pH 8.0. The absorbance at 405 nm was measured in accordance with the reference sample with an inactivated enzyme. One unit (U) of lipase activity was defined as the amount of enzyme releasing 1.0 µmol ρ-nitrophenol for 1 min at 35°С and pH 7.2 [12].
2.10. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was performed in 15% polyacrylamide gel in the presence of SDS on Cleaver Scientific Ltd. OmniPAGE Electrophoresis System CVS10DSYS by the method of Laemmli [17].
2.11. Lipase Zymogram
The enzymes with lipase/esterase activity were detected by zymographic analysis. After non-denaturing PAGE, the gel was treated with 0.02% (w/v) α-naphthyl acetate and 0.05% (w/v) Fast Blue RR salt in 0.05 M Tris-HCl buffer with pH 7.2, revealing bands with lipase activity [18].
3. RESULTS AND DISCUSSION
3.1. Use of ATPS for Lipase Purification
Several factors must be optimized in the development of ATPS for enzymes isolation and purification. The enzyme partition in ATPS depends on the size of the biomolecules, their hydrophobicity, the ionic composition of the phases, polymer concentration and on the molecular weight [19]. In the present study, the use of PEG 4000/ Potassium sodium tartrate ATPS was investigated for isolation of lipase produced in solid-state cultivation of Rhizopus arrhizus.
The binodal curve of the system is presented in Fig. (1), showing the concentrations of PEG4000 and potassium sodium tartrate, which allowed the formation of two non-intermixing aqueous phases. Two phases were formed only above the binodal curve. The construction of the binodal curve had important practical implications for the formation and application of the tested ATPS.
The influence of PEG4000 and potassium sodium tartrate concentrations on lipase partition between the two phases of ATPS is presented in Table 1. The parameters, volume ratio (Rv), partition coefficients of lipase activity (Ke) and protein (Kp), selectivity (S) and partitioning yield of the enzyme in the top phase (PYL), were determined in order to characterize the enzyme partition in the system.

| ATPS Components | Rv | Ke | Kp | S |
PYL % |
|
|---|---|---|---|---|---|---|
|
PEG4000 % (w/w) |
Potassium Sodium Tartrate % (w/w) |
|||||
| 12 | 20 | 0.80 | 1.77 | 0.81 | 2.20 | 58.70 |
| 12 | 23 | 0.80 | 3.29 | 0.78 | 4.24 | 72.48 |
| 12 | 26 | 0.80 | 3.28 | 1.49 | 2.20 | 72.40 |
| 12 | 29 | 0.55 | 4.41 | 1.19 | 3.71 | 70.62 |
| 12 | 32 | 0.50 | 3.80 | 1.11 | 3.44 | 65.52 |
| 12 | 35 | 0.45 | 3.41 | 1.04 | 3.28 | 60.62 |
| 30 | 12 | 5.43 | 12.94 | 2.14 | 6.04 | 98.60 |
| 30 | 15 | 4.62 | 9.76 | 7.29 | 1.33 | 97.83 |
| 30 | 18 | 4.29 | 38.26 | 7.57 | 5.05 | 99.39 |
| 30 | 21 | 4.60 | 77.75 | 12.26 | 6.34 | 99.72 |
| 30 | 24 | 3.25 | 53.63 | 8.02 | 6.69 | 99.43 |
| 30 | 27 | 3.15 | 47.25 | 8.10 | 5.83 | 99.33 |
| 12 | 22 | 0.80 | 1.31 | 1.11 | 1.18 | 51.2 |
| 15 | 22 | 1.20 | 46.55 | 2.26 | 20.63 | 98.23 |
| 18 | 22 | 1.54 | 58.94 | 3.30 | 17.83 | 98.91 |
| 21 | 22 | 1.97 | 70.35 | 4.32 | 16.30 | 99.28 |
| 24 | 22 | 3.29 | 71.67 | 6.09 | 11.77 | 99.58 |
| 27 | 22 | 4.00 | 85.19 | 12.89 | 6.60 | 99.71 |
The initial studies were performed with ATPS with a relatively low concentration of PEG4000 – 12% PEG4000/ 20-35% potassium sodium tartrate. The results showed that all ATPSs tested were characterized by the partition coefficient of lipase Ke > 1, indicating that the enzyme was predominantly distributed in the top PEG-rich phase. The partition coefficient of protein Kp had values lower than or equal to one, indicating that protein was distributed approximately equally between the two phases. The ATPS 12% PEG4000/23% potassium sodium tartrate was characterized by the highest selectivity S = 4.24. The highest partitioning yield of lipase activity in the top phase PYL = 72.48% was achieved with the same system.
Fig. (2) shows the change in the purification degree and recovery yield of lipase activity, depending on salt concentration at 12% PEG 4000.
It should be noted that in all ATPSs tested, a purification fold higher than 1.0 was achieved. The highest value was obtained with 12% PEG4000/23% potassium sodium tartrate ATPS. Recovery yield of lipase activity was between 70-100%. The ATPSs tested were characterized by higher values of recovery yields (70-100%) than the values of partitioning yield in the top phase (58-72%), indicating that lipase activation occurred as a result of applying ATPS for isolation and purification. Lipase activation may be explained by the formation of two phases which may influence the surface activation of lipase. The increased activity of lipases in the presence of apolar-aqueous interphases is known as “interfacial activation” [20]. It can be assumed that as a result of the application of ATPS, the hydrophobicity of the system was changed, which meant that the “open” form of the subdomain lid was a predominant structure leading to the significant activation of lipase [20]. Similar activation of lipase was reported by Zhou et al., they achieved 24% extraction efficiency of lipase in the top phase, and 91.0% recovery yield with 15.1% PEG1500/11.6% potassium phosphate ATPS [21]. Duman and Kaya obtained 266% recovery yield of catalase activity using liquid-liquid extraction with TPP, which was explained by enzyme activation during TPP due to structural changes leading to higher catalytic activity [11].
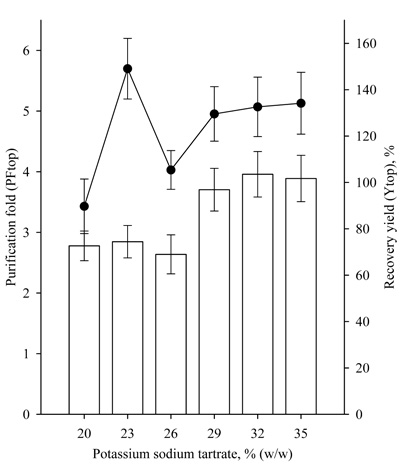
Further studies were conducted with ATPS with a significantly higher concentration of 30% PEG 4000/12-27% potassium sodium tartrate (Table 1). The use of ATPS with a higher PEG 4000 concentration resulted in significantly higher partition coefficients of the enzyme Ke. The highest Ke value 70.75 was observed with 30% PEG4000/21% potassium sodium tartrate system. ATPSs with 30% PEG4000 were characterized by a partitioning yield of lipase activity over 90%. High Ke and partitioning yield values indicated that lipase is distributed in the top polymer containing phase. In addition to the Ke values, the Kp values also increased significantly, indicating that a great part of the proteins was partitioned also in the top phase. Higher partitioning yields also led to significantly higher recovery yields (Fig. 3). The highest 217.71% recovery yield of lipase activity was achieved with 30% PEG 4000/21% potassium sodium tartrate ATPS, and the purification fold was 6.1.
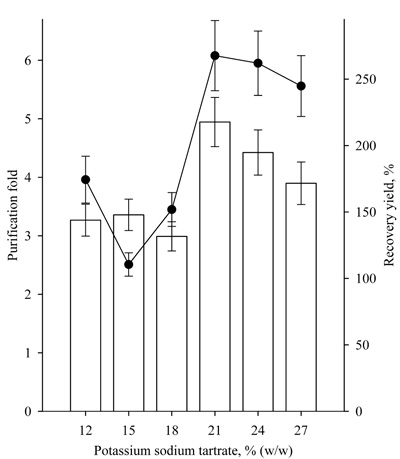
A study on the effect of PEG4000 concentration in the range of 12-27% at 22% potassium sodium tartrate was also conducted. The characteristics of the studied systems are presented in Table 1. It was found that at a PEG4000 concentration of or higher than 15%, the partitioning yields of lipase activity were high, over 90%. Recovery yield of the same systems was observed to be of about 200%, and the purification fold varied about 7 (Fig. 4). Similar results were obtained for lipase purification with PEG/ammonium sulfate ATPS by Anvari, with an increase in the PEG concentration of 10% to 20%. Moreover, the partitioning yield of lipase activity increased from 18.25% to 76.58% in PEG2000/ammonium sulfate ATPS and from 29.73% to 42.15% in PEG4000/ ammonium sulfate ATPS [9].
The results obtained allow the use of PEG 4000/Potassium sodium tartrate ATPS as a mean of developing a downstream process for isolation and purification of lipase with high yield and with the potential for industrial application.
The monitoring of the homogeneity of the isolated lipase by SDS-PAGE and zymographic analysis was not possible due to the influence of PEG4000 in the isolated enzyme preparation, which impeded electrophoretic separation.
3.2. Use of TPP for Lipase Purification
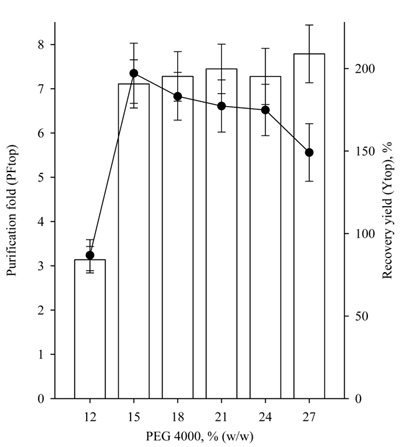
The separation of proteins in TPP system is a complex process, influenced by many factors, including the type and concentration of salt and the organic solvent [10]. Therefore, the effect of t-butanol and ammonium sulfate concentration on the partitioning of lipase from Rhizopus arrhizus was investigated in order to determine the best purification conditions for TPP. The influence of the ratio of crude extract/t-butanol on lipase partitioning by TPP at a constant ammonium sulfate saturation of 30% (w/v) is shown in Fig. (5).

It was found that the recovery yield of lipase activity at the investigated ratios was close to and varied in the range of 70-80%. The highest purification fold was 22.1 ± 1.8 achieved at a crude extract/t-butanol ratio of 1.0:0.5.
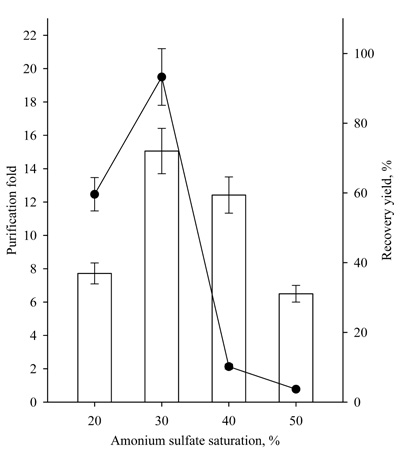
For these reasons, the effect of ammonium sulfate concentration (20-50%) at a fixed ratio of 1.0:0.5 crude enzyme/t-butanol was studied (Fig. 6).
The highest recovery yield of 72.1% ± 6.5 and a purification fold of 19.5 ± 1.7 were achieved at a 30% concentration of ammonium sulfate at a constant crude extract/t-butanol ratio of 1.0:0.5 (v/v).
The precipitation of proteins in the interphase depends not only on the concentration of t-butanol and ammonium sulfate, but also on the pH value. It can be expected that lipase will precipitate with the highest yield at pH values close to pI, because of the weakest solubility of the protein molecule. In addition to the yield of lipase activity, the pH value will also influence the degree of purification because the protein molecules contaminating the lipase enzyme in the crude extract will be characterized by different pI values. The influence of different pH values in the range of pH 6-9 on lipase partitioning is presented in Fig. (7). The study was performed at a fixed crude extract/t-butanol ratio of 1.0:0.5 (v/v) and 30% ammonium sulfate saturation.

The highest yield of 71% ± 6.8 and purification fold of 19.1 ± 1.75 were achieved at pH 7. Higher and lower pH values led to a significant decrease in lipase activity yield and purification degree.
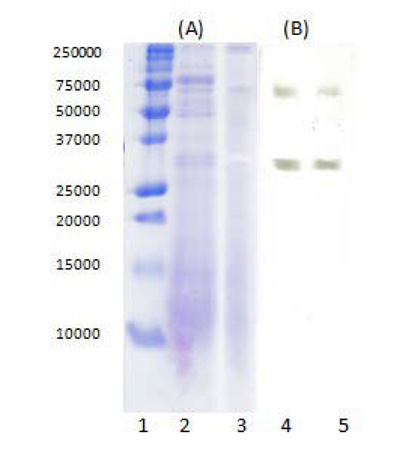
The purification of the tested lipase was monitored by denaturing SDS-PAGE and a zymogram under non-denaturing conditions (Fig. 8). The results from SDS-PAGE (Fig. 8A) showed, that many of the contaminating proteins in the crude enzyme extract were removed as a result of applying TPP (crude extract/t-butanol ratio 1:0.5, ammonium sulfate saturation 30% and pH 7), which explained the high purification fold of 19.1 ± 1.75. By the performed zymographic analysis in non-denaturing conditions (Fig. 8B), two multiple forms of lipase were identified.
CONCLUSION
The methods for the purification of biologically active substances by liquid-liquid extraction such as ATPS and TPP represent an alternative to conventional methods for enzymes’ isolation and purification. They allow the development of downstream processes with high yields, non-toxic eco-friendly materials, low energy costs and no requirement of expensive equipment. As a result of the study, the ATPS PEG4000/ potassium sodium tartrate was found to be a suitable method for isolation and purification of lipase produced by Rhizopus arrhizus in solid state fermentation. When using the system at PEG4000 concentration of or higher than 15%, the lipase enzyme was distributed in the top polymer-rich phase with a partitioning yield of over 90%. The application of PEG4000/ Potassium sodium tartrate ATPS led to significant lipase activation, resulting in a recovery yield of over 100%. At 30% PEG4000/21% Potassium sodium tartrate ATPS, the recovery yield of lipase activity was 217.71% and the purification fold was 6.1. The high lipase activity in the top phase of the ATPS used can be explained by the so-called “interfacial activation”.
TPP with t-butanol/ammonium sulfate based on liquid-liquid extraction is also a suitable method of isolation of lipase. This method is economical, simple, and timesaving. It was found that the best conditions for partitioning of lipase in the interfacial phase were 1.0:0.5 crude enzyme/t-butanol ratio, 30% ammonium sulfate saturation and pH 7. Under these conditions, 71 %± 6.8 recovery yield of lipase activity was achieved, and the purification fold was 19.1 ± 1.75. As compared to the purification with ATPS, the yield of lipase activity was lower but a higher degree of purification was achieved. The presence of two multiple forms of lipase in the crude enzyme extract obtained by solid-state fermentation of Rhizopus arrhizus was established by zymographic analysis.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIAL
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The research was financially supported by the Bulgarian National Science Fund (BNSF), Project 17/25-2017.


