All published articles of this journal are available on ScienceDirect.
Study of Optimum Condition for Rapid Preparation of Thrombin using Russell’s Viper Venom Factor X Activator
Abstract
Background:
The purpose of this study is to investigate a simple method with the optimum condition for rapid thrombin preparation from Cryoprecipitate-depleted Plasma (CDP) using RVV-X in the process.
Methods:
Thrombin preparation from human CDP was studied with the presence of different factors in batch condition including: 1) RVV-X; 2) volume of calcium chloride solution; 3) volume of sodium chloride solution for final extraction; and 4) incubation time. The properties of the prepared sample were analyzed for fibrin clot formation, total protein by Kjeldahl method, thrombin time, molecular weight and protein patterns by SDS-PAGE, and thrombin concentration by coagulation analyzer. The method and process of preparing thrombin and the study of optimum condition for rapidly preparing the highest yield of thrombin from starting CDP 100 ml were introduced.
Results:
The best four conditions were concluded: 1) RVV-X 50 mcg should be present in the process; 2) volume of 0.25 M calcium chloride should be 3 ml; 3) volume of 0.85% sodium chloride for the final protein precipitate extraction should be 10 ml and; 4) no incubation time needed for prothrombin activation process. A solution prepared from the optimum condition showed an obvious band on SDS-PAGE at a molecular weight about 36,000 Da which is our target protein thrombin. The prepared solution had a total protein content of 0.065 g/dl and gave satisfactory results of thrombin time (9 seconds) and fibrin clot formation. The test results of thrombin concentration between the method with and without incubation time were 269.4 and 295.2 IU/ml, respectively.
Conclusion:
This result showed that the method with RVV-X but without incubation time for prothrombin activation (optimum condition) gave the highest yield of thrombin.
1. INTRODUCTION
Thrombin, a serine protease that converts fibrinogen into fibrin in blood coagulation, has played a crucial role in hemostasis and thrombosis. Thrombin is activated from its zymogen, prothrombin, at the site of tissue injury by Factor Xa and its cofactor Factor Va in the presence of phospholipid membrane and calcium [1, 2]. Thrombin is a common hemostatic drug used in surgical practice for over 100 years because of its simplicity and efficacy [3]. Thrombin is present in fibrin glue (also known as fibrin sealant or fibrin tissue adhesive), a plasma-derived hemostatic agent that is composed of thrombin, fibrinogen, and sometimes factor XIII and antifibrinolytic agents. This agent mimics the final steps of the physiological coagulation cascade to form a fibrin clot. Fibrin glue is an effective tissue adhesive used for reducing blood loss in a variety of surgical specialties, and it is biocompatible and biodegradable [4-9].
In addition, thrombin in the form of test reagents has been used for two types of blood coagulation tests: 1) fibrinogen level test; and 2) thrombin time test. Fibrinogen is measured in plasma most commonly using the Clauss method, based on the comparison of thrombin clotting times of dilutions of plasma against a reference plasma with a known level of fibrinogen. Thrombin time (or thrombin clotting time) is a widely performed coagulation assay, which evaluates the ability of fibrinogen to be converted into fibrin after the addition of bovine or human thrombin reagent to the citrated plasma [10-12].
Thrombin is isolated from plasma obtained from bovine or human sources [13]. The plasma is processed through a series of separation and filtration steps followed by incubation of the solution with calcium chloride to isolate and activate prothrombin to thrombin [13], it is also well known that prothrombin can be activated by some components of snake venom to yield thrombin [8]. The solution subsequently undergoes ultrafiltration, vapor heat treatment, solvent-detergent treatment, sterile filtration and freeze-drying [14]. The large-scale production of thrombin from blood plasma (batch size 1200 liters) with a high degree of virus safety can be carried out by these following steps: 1) Isolating prothrombin by the following separation techniques: cryoprecipitation, ion-exchange chromatography (diethyl amino ethyl, DEAE-IEX), heparin affinity chromatography, a second DEAE-IEX step, and Immobilized Metal-Affinity Chromatography (IMAC); 2) Activating prothrombin to thrombin, purifying by Hydrophobic Interaction Chromatography (HIC) and concentrated by ultrafiltration; 3) Reducing viral activity by using substantially different techniques, namely: solvent/detergent (S/D) treatment, pasteurization, and virus filtration (nanofiltration) [15].
Russell’s viper venom factor X activator (RVV-X) is a major procoagulant in Russell’s viper venom. It is a glycoprotein containing 13% carbohydrate with a molecular mass of approximately 93,000 Da, and is composed of a heavy chain (RVV-XH) of molecular mass 58,000 Da and two light chains (RVV-XL) of heterogenous molecular mass 19,000 and 16,000 Da. It directly activates factor X in the final common coagulation pathway, which leads to rapid formation of blood clots [16].
Since purified RVV-X from Russell viper (Daboia russelli siamensis) venom can be prepared at the Snake Bite and Venom Research Unit, Faculty of Medicine, Chulalongkorn University, Thailand [17] and it can be used for prothrombin activation. This study aims to investigate a simple method with the optimum condition for rapid thrombin preparation from human CDP using RVV-X to activate prothrombin in the process.
2. MATERIALS AND METHODS
2.1. Cryoprecipitate-depleted Plasma (CDP)
CDP, the plasma from which cryoprecipitate has been removed, was obtained from Blood Components Production Section, National Blood Centre, Thai Red Cross Society (NBC-TRCS). For the preparation of CDP, a unit of citrated whole blood was spun using a heavy spin in the centrifuge, and the plasma was removed within 6 hours after collection. Fresh Frozen Plasma (FFP) was then prepared by snap freezing the plasma unit at -70 oC and stored at below -30 oC. FFP was slowly thawed overnight at 4°C and centrifuged to separate the cryo-supernatant from the insoluble cryoprecipitate. The insoluble cryoprecipitate was removed and the remaining plasma (CDP) was refrozen.
2.2. Russell’s Viper Venom Factor X Activator (RVV-X)
RVV-X, obtained from Snake Bite and Venom Research Unit, Faculty of Medicine, Chulalongkorn University, Thailand, was purified from crude Daboia russelli siamensis venom by a modification of the procedure of Kisiel et al. (1976) [18] using sequential column chromatography. The specific Factor Xa activity was 1.240 nkat/ng.
2.3. Thrombin Preparation
Thrombin was prepared by a modification procedure of Biggs R. (1972) [19]. The starting volume 100 ml of CDP was diluted to 1000 ml with distilled water, the pH was adjusted to 5.3 with 2% acetic acid and followed by centrifuge. The precipitate was dissolved in 25 ml of 0.85% sodium chloride and the pH was adjusted to 7.0 with 2% sodium carbonate. This was followed by the addition of 0.25 M calcium chloride with or without RVV-X, and incubated for full thrombin formation or without incubation. The coagulated fibrin was removed and acetone was added to the thrombin crude solution (volume 1:1) at room temperature to eliminate electrolytes and to denature some of the protein impurities [20]. The solution was centrifuged to separate the precipitate. The precipitate was finally extracted with 0.85% sodium chloride and followed by centrifuge. The supernatant was collected as thrombin solution and stored in a freezer at a temperature below -20 °C for its stability before the properties were tested.
Thrombin was prepared using the procedure described above in different conditions varying 4 related factors including: 1) RVV-X; 2) 0.25 M calcium chloride volume; 3) incubation time; and 4) 0.85% sodium chloride final extraction volume. The study compared different conditions step by step to determine the optimum preparing condition as the following methods: M1.0 vs. M2.0; M2.0 vs. M2.1; M2.0 vs. M2.2; and M2.0 vs. M2.3 (Table 1). The prepared thrombin was then further analyzed by different methods for the following properties: 1) fibrin clot formation; 2) total protein; and 3) Thrombin Clotting Time (TT). The prepared thrombin from two optimum conditions was analyzed and compared for: 1) Molecular Weight (MW) and protein patterns; and 2) thrombin concentration.
| Test Factors | Method | Conditions | |||
|---|---|---|---|---|---|
| - | - | 0.25 M CaCl2 Volume (ml) | RVV-X (mcg) |
0.85% NaCl Final Extraction Volume (ml) | Incubation Time (hours) |
| 1) RVV-X | M1.0 | 3 | - | 25 | 2 |
| M2.0 | 3 | 50 | 25 | 2 | |
| 2) 0.25 M CaCl2 | M2.0 | 3 | 50 | 25 | 2 |
| Volume | M2.1 | 1.5 | 50 | 25 | 2 |
| 3) 0.85% NaCl Final | M2.0 | 3 | 50 | 25 | 2 |
| Extraction Volume | M2.2 | 3 | 50 | 10 | 2 |
| 4) Incubation Time | M2.2 | 3 | 50 | 10 | 2 |
| - | M2.3 | 3 | 50 | 10 | - |
2.4. Fibrin Clot Formation
The prepared thrombin was preliminarily tested for its activity by observation of fibrin clot formation after mixing with cryoprecipitate (volume 1:1) obtained from Blood Components Production Section, NBC-TRCS.
2.5. Total Protein Assay
Kjeldahl method (determination of nitrogen by sulfuric acid digestion) was used for total protein assay of prepared thrombin according to the method described by European Pharmacopoeia 8.0 (2014) [21].
2.6. Thrombin Clotting Time
The prepared thrombin was diluted to 1:50 with distilled water. One hundred microliters of diluted thrombin was added to 100 microliters of fresh plasma in the test tube at 37 °C. The tube was gently shaken and tilted back and forth. The time taken for the first appearance of a fibrin clot was recorded.
2.7. Molecular Weight and Protein Patterns
The Molecular Weight (MW) of the prepared thrombin was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using standard protein molecular weight markers.
2.8. Thrombin Concentration
The prepared thrombin was determined by the automated coagulation analyzer (Sysmex® CA-560) compared to Japanese Pharmacopoeia Reference Standard Thrombin (690 units/ampoule, Control: THR0104) from the Society of Japanese Pharmacopoeia, for its activity in the International Units (IU/ml).
3. RESULTS AND DISCUSSION
Thrombin solution was prepared in different conditions and the properties of produced thrombin were compared step by step, in order to study the effects of four related factors in the thrombin preparation process.
3.1. RVV-X
Prothrombin is the coagulation proenzyme present in the highest concentration blood (0.07-0.1 mg/ml) and was recognized very early as a prime contributor to the blood coagulation process [22]. By the common pathway, activated factor X (factor Xa) along with its cofactor (factor V), tissue phospholipids, platelet phospholipids and calcium forms the prothrombinase complex which converts prothrombin into thrombin [23]. In our study, factor X has been enzymatically activated by a coagulant protein (heterotrimeric metalloproteinase) [24] in Russell's viper venom (RVV-X) to help make the conversion of prothrombin more complete than adding calcium to the plasma alone.
The first study was conducted on the preparation of thrombin using Method M 1.0 and M 2.0 to compare the presence and absence of RVV-X in the preparation process. Thrombin was prepared from CDP (or cryo-supernatant), which is rich in prothrombin complex and has a low level of fibrinogen [25, 26], by the procedure described in 2.3. From starting volume 100 ml of CDP, we obtained about 25 ml of thrombin solution in the preparation batch. The solution was slightly turbid with some small particles due to no filtration step in the preparation process.
The preliminary test confirmed that there was thrombin in the prepared solutions, which can be observed from the reactions between thrombin and fibrinogen in cryoprecipitate to make the fibrin clot formation (Fig. 1).

Total protein content analyzed by Kjeldahl method showed that the thrombin solution of Method M2.0 had higher protein content than of Method M1.0, as well as the shorter thrombin time of Method M2.0 than of Method M1.0. At this step, we concluded that the use of RVV-X in Method M2.0 resulted in a greater amount of thrombin than that of Method M1.0 without RVV-X (Table 2).
| Condition | Fibrin Clot Formation | Total Protein (g/dl) | Thrombin Time (s) |
|---|---|---|---|
| M1.0 (no RVV-X) | Fibrin clot formed | 0.036 | 18.0 |
| M2.0 (used RVV-X) | Fibrin clot formed | 0.052 | 14.0 |
3.2. Calcium Chloride Volume
The reaction of coagulant protein on factor X is proteolytic, has an absolute requirement for Ca (II), and proceeds at physiological pH [27]. In this study, 0.25 M calcium chloride was reduced by half to 1.5 ml to see how it affects the preparation of thrombin.
At the half-dose of 0.25 M calcium chloride (Method M2.1), the total protein content of thrombin solution was lower than Method M2.0, and the thrombin time of Method M2.1 was twice longer than Method M2.0, indicating that the amount of thrombin prepared by Method M2.1 was much lower than of Method M2.0. In this step, it was suggested that the thrombin preparation process should have a sufficient amount of calcium in order to maximize the conversion of prothrombin (Table 3). This result corresponded to the previous study which indicated that prothrombin concentrates incubated with near-physiological levels of calcium appeared to correct the abnormal APTT to an increasing degree as the calcium concentration was increased [28].
| Condition | Fibrin Clot Formation | Total Protein (g/dl) | Thrombin Time (s) |
|---|---|---|---|
| M2.0 (CaCl2 = 3 ml) | Fibrin clot formed | 0.052 | 14.0 |
| M2.1 (CaCl2 = 1.5 ml) | Fibrin clot formed | 0.022 | 29.0 |
3.3. Sodium Chloride Final Extraction Volume
For the next study, the different volume of 0.85% sodium chloride solution used to dissolve the final protein precipitate was compared between 25 ml (100%) in Method M2.0 and 10 ml (40%) in Method M2.2, to determine whether the sodium chloride extraction volume affected the properties of prepared thrombin or not.
The total protein content of Method M2.2 thrombin solution was higher than that of Method M2.0, and the thrombin time of Method M2.2 thrombin solution was nearly twice shorter than that of Method M2.0. These results indicated that thrombin concentration of Method M2.2 was highly concentrated, therefore resulted in higher activity than Method M2.0 (Table 4).
| Condition | Fibrin Clot Formation | Total Protein (g/dl) | Thrombin Time (s) |
|---|---|---|---|
| M2.0 (NaCl = 25 ml) | Fibrin clot formed | 0.052 | 14.0 |
| M2.2 (NaCl = 10 ml) | Fibrin clot formed | 0.063 | 8.0 |
3.4. Incubation Time
After the first three studies on RVV-X, calcium and sodium chloride final extraction volume, the most appropriate method for producing thrombin was Method M2.2 (using RVV-X 50 mcg, 0.25 M calcium chloride 3 ml, 0.85% sodium chloride volume 10 ml for final extraction, and incubation time 2 hours) as it gave the highest total protein and lowest thrombin time. The next step was to compare the incubation time factor between Method M2.2 (incubation time 2 hours) and M2.3 (no incubation time) to determine whether thrombin can be generated without incubation time. Theoretically, the RVV-X, a factor X activator, is capable of promptly converting prothrombin into thrombin [29]. Therefore, it was expected that Method M2.3 would result in thrombin amount not different from that of M2.2.
The results showed that the total protein content of thrombin solutions including the thrombin time of Method M2.2 and M2.3 was very similar. However, the preparation process of Method M2.3 was more convenient as it did not require incubation time up to 2 hours (Table 5).
| Condition | Fibrin Clot Formation | Total Protein (g/dl) | Thrombin Time (s) |
|---|---|---|---|
| M2.2 (incubation time 2 hours) | Fibrin clot formed | 0.063 | 8.0 |
| M2.3 (no incubation time) | Fibrin clot formed | 0.065 | 9.0 |
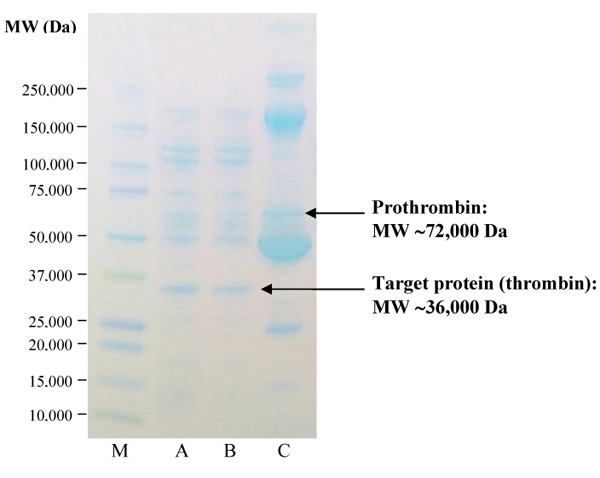
The MW of the prepared thrombin from two optimum conditions (Method M2.2 and M2.3) and diluted CDP were determined by SDS-PAGE using standard protein molecular weight markers (Fig. 2). From SDS-PAGE results, CDP contained several proteins including 72,000 Da prothrombin (lane C). Upon activation with calcium and RVV-X, 36,000 Da protein band which is the size of thrombin protein, appeared (lane A and B) [30, 31]. Thrombin bands on the digest of Method M2.2 (lane A) and Method M2.3 (lane B) had a similar intensity even without incubation process in Method M2.3 due to the ability of RVV-X in activated prothrombin into thrombin promptly.
At this stage, thrombin concentration has been tested using a coagulation analyzer by comparison with the standard thrombin, and reported as a thrombin unit. It was found that the thrombin concentration of Method M2.2 and M2.3 was 269.4 and 295.2 IU/ml, respectively, which indicated that Method M2.3 was the most rapid preparation method to produce thrombin and gave the highest yield of thrombin as well (Table 6).
| Condition | SDS-PAGE | Thrombin Concentration (IU/ml) |
|---|---|---|
| M2.2 (incubation time 2 hours) | Thrombin band appeared at about 36,000 Da |
269.4 |
| M2.3 (no incubation time) | Thrombin band appeared at about 36,000 Da |
295.2 |
CONCLUSION AND RECOMMENDATIONS
The method and process of preparing thrombin and the study of optimum condition for rapid preparing the highest yield of thrombin from starting CDP 100 ml were introduced. The best four conditions were concluded: 1) RVV-X 50 mcg should be present in the process; 2) volume of 0.25 M calcium chloride should be 3 ml; 3) volume of 0.85% sodium chloride for the final protein precipitate extraction should be 10 ml and 4) no incubation time needed for prothrombin activation process. The solution prepared from the optimum condition showed an obvious band on SDS-PAGE at a molecular weight about 36,000 Da which is our target protein thrombin. The prepared solution had a total protein content of 0.065 g/dl and gave satisfactory results of thrombin time (9 seconds) and fibrin clot formation. Testing comparison of thrombin concentration between the method with and without incubation time was carried out on a coagulation analyzer, and the results were 269.4 and 295.2 IU/ml, respectively. This result showed that the method with RVV-X but without incubation time for prothrombin activation (optimum condition) gave the highest yield of thrombin.
The prepared thrombin should be further purified with suitable affinity chromatography for higher purity and potency in the next study.
LIST OF ABBREVIATIONS
| APTT | = Activated Partial Thromboplastin Time |
| Ca | = Calcium |
| CaCl2 | = Calcium Chloride |
| CDP | = Cryoprecipitate-depleted Plasma |
| Da | = Dalton (s) |
| DEAE-IEX | = Diethyl Amino Ethyl Ion-Exchange Chromatography |
| dl | = Deciliter (s) |
| FFP | = Fresh Frozen Plasma |
| g | = Gram (s) |
| HIC | = Hydrophobic Interaction Chromatography |
| IMAC | = Immobilized Metal-Affinity Chromatography |
| IU | = International Unit (s) |
| M | = Molar |
| ml | = Milliliter (s) |
| MW | = Molecular Weight |
| NaCl | = Sodium Chloride |
| NBC-TRCS | = National Blood Centre, Thai Red Cross Society |
| RVV-X | = Russell’s Viper Venom Factor X Activator |
| s | = Second (s) |
| S/D | = Solvent/Detergent |
| SDS-PAGE | = Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| vs. | = Versus |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research was approved by the Institutional Review Committee of the National Blood Centre, Thai Red Cross Society.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Antiserum and Standard Cells Production Section of NBC-TRCS for providing equipment and instruments for our research with excellent facilities.
We also would like to extend our sincere thanks to the Quality Control Section of NBC-TRCS for testing results in this study.


