All published articles of this journal are available on ScienceDirect.
Productivity and Biodiesel Quality of Fatty Acids Contents from Scenedesmus obliquus in Domestic Wastewater Using Phototrophic and Mixotrophic Cultivation Systems
Abstract
Background:
Microalgae remove nutrients from wastewater with the possibility of grow in mixotrophic and heterotrophic cultures. However, the effluent quality can modify the profile of fatty acids and biodiesel quality.
Methods:
Phototrophic and mixotrophic (light / dark; 12/12 h) cultures of Scenedesmus obliquus on domestic wastewater (WW) and Artificial Wastewater (AW) was carried out to evaluate the lipid accumulation and fatty acid methyl esters profile. The microalgae was first cultivated in an enriched medium (90 mg N-NH4 L-1) and subsequently under nitrogen limitation (30, 20 and 10 mg N L-1) using a two-stage process for both culture media.
Results:
A higher cell density in enriched AW medium was obtained in phototrophic and mixotrophic culture of 19 x 106 cell mL-1 and 20 x 106 cell mL-1, respectively; than for WW (13 x 106 cell mL-1 and 14 x 106 cell mL-1, respectively). The nitrogen limitation (from 90 to 20 mg N L-1) for AW increased the lipid content by 5.0% and 17.28% under phototrophic and mixotrophic conditions, respectively and only 5% for WW in mixotrophic culture.
Conclusion:
The high Cetane Number (CN) show a positive correlation with high Saturated Fatty Acids (SFA) content and negative correlation with the Degree of Saturation (DU), suggesting a good ignition of fuel. The Cold Filter Plugging Point (CFPP) (-6.02 to -8.45 °C) and Oxidative Stability (OS) (3.53 - 6.6 h) propose to Scenedesmus obliquus as a candidate in the production of biodiesel and potential application for an integral urban wastewater treatment system.
1. INTRODUCTION
In recent years, environmental issues (climate change) in relation to the effects caused by the high consumption of fossil fuels and the progressive depletion of this resource in many countries have motivated new research aimed at the
exploration of new renewable energy sources such as the use of microalgal biomass to obtain lipids and synthesis of biodiesel [1]. Microalgae have shown important properties such as high photosynthetic efficiency, high rates of lipid accumulation and high biomass production. Some of the microalgae species reserve high lipid content (above 70%) under conditions of environmental stressors such as limitation of essential nutrients as nitrogen, phosphate and some metals.
Nitrogen limitation changes cellular carbon flux from protein synthesis to lipid synthesis, resulting in increases of 20% to 40% in microalgae [2, 3]. However, high lipids contents produced under nutrient limitation conditions are usually associated with low algal biomass productivity. A replenishing nitrogen source is generally necessary to maintain a high cell growth rate and achieve high cell density. Producing sufficient biomass with enhanced lipids contents can be done using a two-stage culture strategy [4]. In this strategy, an alga is first grown under nutrient-sufficient conditions to allow maximum cell density, and then deprived of certain nutrients to trigger lipids accumulation [4, 5].
Another factor that contributes to the increase in the content of lipids and algal biomass for purposes of biodiesel production is culture in the presence of carbon sources. The algae can consume both sources of organic carbon (sugars) and inorganic carbon (CO2), therefore, it has been proposed to combine the algal culture in urban wastewater treatment systems, with the opportunity to produce a renewable energy (Biodiesel) and be able to reduce costs for both processes [6].
There are many applications that in recent years have been attributed to algal culture and in the search to minimize the costs of biomass production studies have turned their attention to the use of industrial and urban wastewater, where the goal is to reduce costs and utilization of the content of nitrogen and phosphorus present in the effluents, which suggests that the cultivation of algae in wastewater does not generate additional contamination when the biomass is harvested, making the treatment system more attractive than the activated sludge systems [7]. Recently the debate on the use of algae-wastewater system for production of raw material for biofuel production focuses on evaluating the algal biochemical composition as it is not completely satisfactory for the biodiesel synthesis. Although in experiments with algae strains a great vitality has been observed in wastewater, the variations in the biochemical composition are dependent on the algal species and environmental conditions [8].
Therefore, the objective of the present work was to evaluate the profile of fatty acids by Scenedesmus obliquus, estimating the quality of biodiesel produced in phototrophic and mixotrophic culture mode using domestic wastewater as a source of nutrients and organic carbon.
2. MATERIALS AND METHODS
2.1. Strain Selection and Culture Media
The microalgae Scenedesmus obliquus was obtained from the Biology Laboratory and microalgae culture collection of the Centro de Investigación Científica y de Educación Superior de Ensenada, Baja California (CICESE). The microalgae Scenedesmus obliquus was chosen due to its ability to grow in wastewater and its high N and P removal efficiencies. Basic growth culture medium composition was similar to that of primary treatment effluent from the urban wastewater treatment plants [7]: 7 mg NaCl; 4 mg CaCl2; 2 mg MgSO4∙7H2O; 15 mg KH2PO4; 115.6 mg NH4Cl; 100 mg glucose; all dissolved in 1 L drinking water. Trace metals and vitamins were added according to guidelines for f/2 medium [9]. Culture conditions were 28 ± 1 °C with a 100 µE m–2 s–1 light intensity. During the 1 month acclimatization period, the culture was transferred to fresh media every seven days.
2.2. Experimental Design
In all experiments, Scenedesmus obliquus was cultured in cylindrical bioreactors made of transparent Polyethylene Terephthalate (PETE) with a 3 L maximum capacity. Reactors were pre-washed with a chlorine solution (0.5%) to prevent bacterial contamination. All treatments were run in triplicate at a constant temperature of 28 ± 1 °C, an initial cell density of 2×106 cell mL-1 and aeration at 0.4 L L-1 min-1. In the phototrophic treatments, continuous light (100 µE s-1 m-2) was provided using cool white fluorescent lamps and for the mixotrophic treatments, the same light intensity was applied in 12/12 h light/dark cycles.
A two-stage nitrogen reduction process was used. In the first stage, S. obliquus was grown in 1 L of medium containing 90 mg-N L-1 (nitrogen sufficiency). When the exponential growth phase ended, 1 L of fresh medium was added to the dilution until reaching 2 L of media containing 10, 20 y 30 mg L-1 N-NH4. All treatments were run in triplicate for the two culture media: artificial wastewater and urban wastewater (Table 1). and the nitrogen concentration was determined according to the standard methods of water and wastewater [10].
| Culture Media | Photoperiod / Growth Mode | Nitrogen Reduction Levels |
|---|---|---|
| Artificial Wastewater (AW) |
Photoautotrophic | 90 mg L–1 |
| From 90 to 30 mg L–1 | ||
| From 90 to 20 mg L–1 | ||
| From 90 to 10 mg L–1 | ||
| Mixotrophic (12/12 h) | 90 mg L–1 | |
| From 90 to 30 mg L–1 | ||
| From 90 to 20 mg L–1 | ||
| From 90 to 10 mg L–1 | ||
| Urban Wastewater (WW) |
Photoautotrophic | 90 mg L–1 |
| From 90 to 30 mg L–1 | ||
| From 90 to 20 mg L–1 | ||
| From 90 to 10 mg L–1 | ||
| Mixotrophic (12/12 h) | 90 mg L–1 | |
| From 90 to 30 mg L–1 | ||
| From 90 to 20 mg L–1 | ||
| From 90 to 10 mg L–1 |
The domestic effluents were collected at the discharge from the aeration tanks of three wastewater treatment plant in the municipality of Ciudad del Carmen, Campeche; Mexico. The characteristics of the samples for the three plants are listed in Table 2. As the content of N-NH4 of the urban effluents was considered with variations in months for cultivating microalgae, the NH4 concentration was adjusted to 90 mg L-1 and 30, 20 y 10 mg L-1 in order to reach a similar value for all the treatments.
| Parameters | Units | Wastewater Treatment Plants Effluents | ||
|---|---|---|---|---|
| WPT1 | WPT2 | WPT3 | ||
| Temperature | °C | 26.3 ± 0.2 | 27.0 ± 0.1 | 27.6 ± 0.01 |
| pH | - | 7.96 | 7.47 | 7.10 |
| Total phosphorus | mg L-1 | 19.64 ± 0.1 | 3.75 ± 0.2 | 4.22 ± 0.02 |
| Total nitrogen | mg L-1 | 99.13 ± 0.02 | 18.64 ± 0.1 | 3.10 ± 0.1 |
| N-NH4 | mg L-1 | 90.75 ± 0.03 | 17.06 ± 0.02 | 2.84 ± 0.02 |
| N-NO3 | mg L-1 | 8.29 ± 0.01 | 1.81 ± 0.01 | 0.32 ± 0.03 |
| N-NO2 | mg L-1 | 0.08 ± 0.1 | 0.013 ± 0.2 | 0.002 ± 0.2 |
| N/P ratio | - | 5.04 | 4.97 | 0.73 |
| Fats and oil | mg L-1 | 20.26 ± 1.2 | 8.03 ± 2.2 | 10.75 ± 1.8 |
| BOD | mg L-1 | 212 ± 0.8 | 28 ± 1.2 | 43 ± 0.5 |
| TSS | mg L-1 | 117 ± 0.5 | 27 ± 0.2 | 40 ± 0.3 |
2.3. Fatty Acid Extraction and Composition Analysis
At the end of each treatment, approximately 1 L culture was collected from the bioreactors and centrifuged at 4500 rpm and 14 °C for 15 min. The recovered biomass was frozen at -4.0 °C for 48 h, and then lyophilized for 3-5 days; the resulting dry biomass was stored at 0 °C. Total lipids were extracted following the dry extraction procedure described by Zhu et al. [11] and Feng et al. [12], and then quantified using the method of Pande et al. [13] using a tripalmitin standard (99%, Sigma-Aldrich).
The transesterification of fatty acids was according to the methods by Sato and Murata [14]; and Canedo-Lopez et al. [5]. The FAMEs profiles were generated with a Gas Chromatographer (GC) (Agilent Technology 7890). One microliter of the FAME-hexane solution was injected into the GC equipped with a Flame Ionization Detector (FID) and separation was done in a DB-23 column (60 m length, 0.32 mm ID, 0.25 µm thick) with helium as the vehicle. Injector and detector temperature was 250 °C. A five-step temperature program was run: 120 ºC for 5 min; 10 °C min–1 increases until reaching 180 °C; 180 °C for 30 min; 10 °C min–1 increases until reaching 210 °C; and 210 °C for 21 min [5]. A calibration curve was prepared for all FAMEs by injecting known concentrations of an external standard mixture containing 37 FAMEs (Supelco, Bellefonte, PA, USA); the correlation coefficient was equal to or greater than 95% in all cases.
2.4. Productivity and Evaluation of Fatty acid Quality for Biodiesel
Lipid productivity PL (grams of lipids per liter of culture per day) was calculated using equation (1) [15, 16].
 |
(1) |
Where w is the lipid content as a weight fraction (g lipid per g dry biomass); and X is biomass concentration (g dry biomass per mL culture). Thus, Δ(wX) represents the lipids accumulated from inoculation (time t1) to harvest (time t2), which occurs in time Δt.
The specific growth rate µ in the logarithmic growth phase was defined as (equation 2):
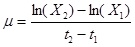 |
(2) |
Where t1 and t2 are cultivation times during the logarithmic phase.
Biodiesel quality (fuel characteristics) such as Saponification Value (SV), Cetane Number (CN), Iodine Value (IV), Long Chain Saturated Factor (LCSF) and Col Filter Plugging Point (CFPP) were determined based on the fatty acid composition of the microalgal using empirical equation described by Vidyashankar et al. [17] and Guldhe et al. [18].
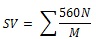 |
(3) |
 |
(4) |
 |
(5) |
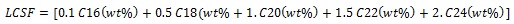 |
(6) |
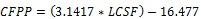 |
(7) |
Where D, M and N denote the number of double bonds, molecular mass and % mass fraction of each fatty acid component, respectively.
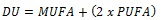 |
(8) |
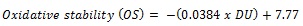 |
(9) |
Where, DU is the degree of unsaturated, calculated from the fatty acid profile [18, 19]. The properties of synthesized biodiesel were compared with the specifications given by ASTM 6751 and EN14214 standards.
2.5. Statistical Analysis
An analysis of covariance (ANCOVA; P ≤ 0.05) was applied to measure the effects of the manipulated variables (nitrogen limitation, photoperiod) on the response variables (specific growth rate and lipids productivity). The Tukey test (HSD) was applied when results exhibited significant differences.
3. RESULTS AND DISCUSSION
3.1. Growth and Nutrients Removal
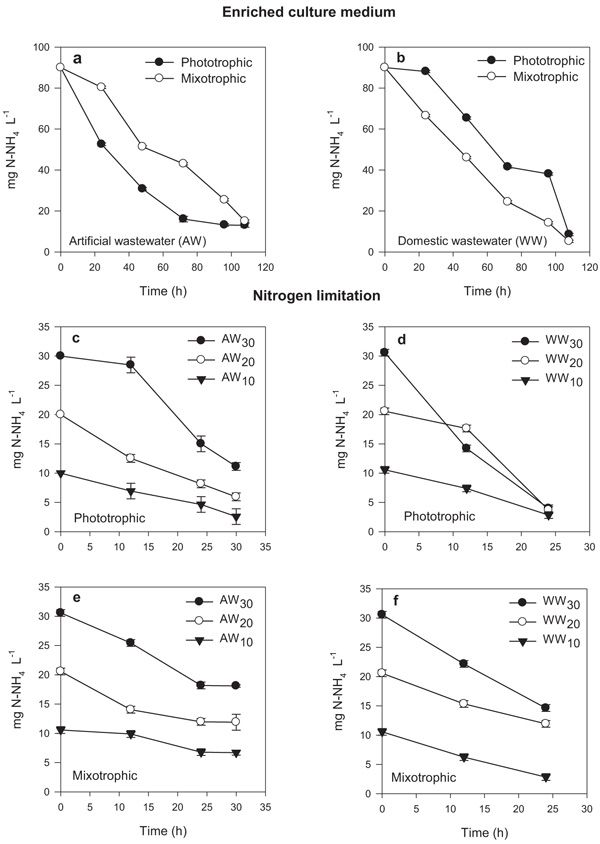
The growth kinetics obtained from the microalgae S. obliquus during the culture period showed favorable increases in cell density for both culture media: artificial and urban wastewater (Fig. 1). The growth for each culture medium showed significant differences (P ≤ 0.015); where the highest growth was observed mainly in culture with artificial wastewater with respect to the urban wastewater medium (Fig. 1). The growth rate (μ) decreased in urban wastewater medium, similar to that reported by several investigations (Table 3), which is attributed to competition for other microorganisms present in the effluents (bacteria and protozoa), as well as the different forms of bioavailable nutrients [7, 20].
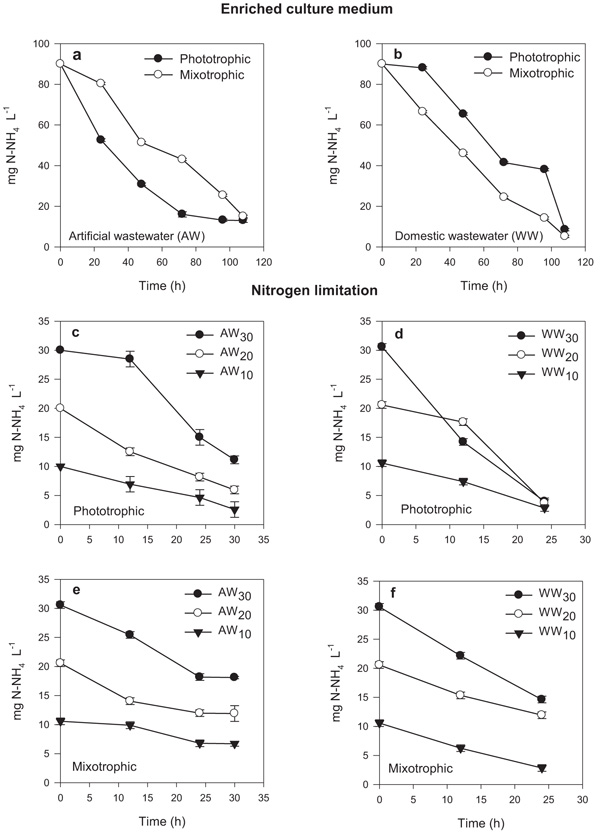
In particular, phototrophic and mixotrophic growth in similar culture medium (artificial and urban wastewater) did not present significant differences (P ≥ 0.121), suggesting that S. obliquus presented the ability to change its metabolism from phototrophic to mixotrophic (Figs. 1a, b). The maximum cell density under phototrophic and mixotrophic culture obtained in artificial wastewater was 19 x 106 cell mL-1 and 20 x 106 cell mL-1, respectively; while in urban wastewater culture, it was 13 x 106 cell mL-1 and 14 x 106 cell mL-1, respectively. This shows the capacity of S. obliquus to adapt and grow in urban wastewater, as well as use inorganic and organic carbon sources in mixotrophic culture systems [5, 7, 21]. On the other hand, the treatments under nitrogen limitation (Figs. 1c, d, e, f) growth showed significant differences during nitrogen variation (30, 20 and 10 mg L-1) for both phototrophic and mixotrophic systems. The lowest cell density was for those mixotrophic cultures in urban wastewater (Fig. 1f).
A probable explanation can be attributed to the fact that wastewater contains high levels of carbon, useful for some microalgae with the capacity to grow in a mixotrophic or heterotrophic environment, however, this organic carbon that has been previously degraded by bacteria under aerobic conditions, results inert and recalcitrant, becoming not available for consumption by microalgae. In the present study, the wastewater was not sterilized, which suggested that bacteria and the unavailable forms of carbon sources in the effluent were probably not favorable for the growth of S. obliquus under mixotrophic conditions [5, 22, 23]; suggesting that the rate of growth and removal of nutrients depends greatly on the organic loads present in the urban wastewater effluents.
A similar tendency was observed in the nitrogen removal for S. obliquus (Fig. 2) in medium enriched for both artificial and urban wastewater, where the efficiency was 90% of nitrogen removed in both phototrophic and mixotrophic conditions (Figs. 2a, b). Studies have reported that it is possible to achieve efficient nitrogen removal in wastewater treatments, similar to that reported in the present study [21, 24]. However, under nitrogen limitation at 30, 20 and 10 mg L-1, it was observed that 80% of nitrogen was removed in the photoautotrophic system (figure c and d); while in mixotrophic culture, the nitrogen removal was not greater than 50% (Figs. 2e, f).
Based on the results of growth and nitrogen removal under nitrogen limitation, it was possible selected the treatment with nitrogen reduction of 90 mg N-NH4 L-1 at 20 mg L-1 with the objective of evaluating the fatty acid profile and quality of biodiesel.
3.2. Productivity and Fuel Quality of S. obliquus-Based Biodiesel
The analysis of productivity and fatty acids profile presented in this study was based on the Two-Stage method at a limiting concentration of 20 mg N-NH4 L-1. The lipids accumulation had an increase of 5.24% to 17.28% nitrogen limitation under phototrophic and mixotrophic conditions, respectively, using Artificial Wastewater (AW). While cultures with urban wastewater (WW) did not show a significant increase in lipids content for one autotrophic system, unlike a 5% increase in mixotrophic culture (Table 3).
| Parameters | Culture Medium | Treatments | |||
|---|---|---|---|---|---|
| Phototrophic | Mixotrophic | ||||
| WA | WW | WA | WW | ||
| µ (d-1) | Enriched culture | 0.643 | 0.541 | 0.389 | 0.296 |
| Nitrogen limitation | 0.425 | 0.179 | 0.354 | 0.225 | |
| g lipids/g biomass (% w/w) |
Enriched culture | 19.9 | 20.8 | 15.8 | 17.4 |
| Nitrogen limitation | 21.0 | 19.0 | 19.1 | 18.3 | |
| Productivity (mg lipids L-1 d-1) |
Enriched culture | 26.49 | 23.41 | 19.24 | 16.31 |
| Nitrogen limitation | 35.35 | 41.61 | 37.21 | 28.18 | |
Although the values of lipid production (% w / w) were low in WW compared to those obtained in AW, the results suggest that the microalga had the capacity to increase the lipid content under the mixotrophic condition and nitrogen limitation in urban wastewater. Mandal and Mallick [25] reported for S. obliquus a productivity (7. 14 mg lipid L-1 d-1) under phototrophic condition lower than that reported in the present study (Table 3). While Liang et al. [26] reported high productivity ranges of 11.6 - 58.6 mg lipid L-1 d-1 for S. obliquus under mixotrophic condition.
This increase in productivity was also observed in the present studies when passing the cultures from a phototrophic to mixotrophic condition. Although studies have reported that productivity for S. obliquus may decrease (8 mg lipid L-1 d-1) in cultures using secondary effluents [27], this was not observed in the present study, suggesting that wastewater can be proposed as a culture medium to substitute the conventional culture media, given that it is possible to achieve high productivity in phototrophic and mixotrophic conditions (Table 3); offering an opportunity for microalgae technology in the removal of nutrients from wastewater treatment plants effluents, with the additional advantage of obtaining biomass with a favorable lipid content for the synthesis of biodiesel. However, some factors must be considered when using wastewater, such as organic load, organic and inorganic carbon content, nitrogen and phosphorus content, since the quality of the effluents varies according to the form of operation and technology used for the treatment of wastewater that could affect the biochemistry (carbohydrates, proteins and fatty acid profile) of the algal biomass.
The fatty acid methyl ester composition of biodiesel obtained by S. obliquus is shown in Table 4. The Behenic acid (C22:0) and Myristic acid (C14:0) were the predominant Saturated Fatty Acids (SFA) contributing to phototropic culture to reach the 64.19% - 69.10% of SFA and for mixotrophic culture of 48.6% - 62.30% of SFA (Table 5). While the Monounsaturated Fatty Acid (MUFA) predominant was the Eicosenoic acid (C20: 1) contributing to 25% -34.75% of the MUFA present in the algal biomass (Table 4). It is a fact that the profile and composition of oil obtained by S. obliquus cultivated in domestic residual water, showed no differences to artificial wastewater cultures, suggesting that the fatty acid profile for S. obliquus was not affected; which proposes domestic wastewater as a good substitute growing medium for the production of algal biomass.
| FAME | Artificial Wastewater (AW) | Domestic Wastewater (WW) | ||||||
|---|---|---|---|---|---|---|---|---|
| Enriched Culture | Nitrogen Limitation | Enriched Culture | Nitrogen Limitation | |||||
| Phot | Mixotr | Phot | Mixotr | Phot | Mixotr | Phot | Mixotr | |
| Myristic acid (C14:0) | 10.62 | 14.12 | 20.40 | 12.51 | 19.98 | 19.44 | 24.88 | 20.84 |
| Palmitic acid (C16:0) | 9.31 | 0.95 | 8.51 | 8.35 | 7.33 | 8.23 | 7.23 | 7.48 |
| Arachidic acid (C20:0) | 11.61 | 8.72 | 7.21 | 8.23 | 11.74 | 7.21 | 7.48 | 6.63 |
| Behenic acid (C22:0) | 17.14 | 15.69 | 14.71 | 19.49 | 11.44 | 13.20 | 13.55 | 13.03 |
| Palmitoleic acid (C16:1) | 8.73 | 8.55 | 6.71 | 7.68 | 7.88 | 7.06 | 6.52 | 6.81 |
| Oleic acid (C18:1) | 0.63 | 2.14 | 0.83 | 1.80 | 1.03 | 1.26 | 1.84 | 2.16 |
| Eicosenoic acid (C20:1) | 17.11 | 16.93 | 15.96 | 17.26 | 15.82 | 13.78 | 14.17 | 12.67 |
| Erucic acid (C22:1) | 9.13 | 11.47 | 9.34 | 8.00 | 9.60 | 11.30 | 8.53 | 10.28 |
| Linoleic acid (C18:2) | 9.64 | 12.01 | 9.83 | 8.43 | 9.13 | 10.28 | 8.95 | 9.56 |
| Linolenic acid (C18:3) | 6.07 | 9.39 | 6.48 | 8.19 | 6.01 | 8.20 | 6.82 | 10.51 |
| Culture Mode | Culture Medium | SAF (% wt) | MUFA (% wt) | PUFA (% wt) | LCSF (% wt) |
|---|---|---|---|---|---|
| phototrophic | AW | 69.10 | 25.83 | 5.00 | 2.98 |
| WW | 64.19 | 25.00 | 10.80 | 3.33 | |
| Mixotrophic | AW | 48.60 | 34.75 | 16.63 | 2.77 |
| WW | 62.30 | 25.30 | 12.4 | 2.55 |
A subject of interest is the content of Saturated Fatty Acids (SFA), monosaturated (MUFA) and polyunsaturated (PUFA) present in the oil since these have a high impact on the quality of biodiesel. In the present study, the high proportion of SFA compared with MUFA and PUFA in S. obliquus contributes to an increase in oxidative stability (Table 5). An analysis of oil containing high concentrations of PUFA and MUFA on SFA tends to originate a biodiesel with high iodine value, a condition that determines the generation of a biodiesel that is oxidation prone; however, this was not observed in domestic residual water cultures (WW) and Artificial Wastewater (AW) (Table 5). Therefore, the quality parameters evaluated as the cetane number and saponification value were related to the saturation of FAME in both cultures of S. obliquus in AW and WW.
The parameters determined in the present study that define the biodiesel quality were: Cetane Number (CN), iodine value (IV), Cold Filter Plugging Point (CFPP); Saponification Value (SV), Degree of Unsaturation (DU) and, Oxidative Stability (OS) for autotrophic and mixotrophic conditions in AW and WW medium (Table 6).
Cetane Number (CN) is the main indicator of biodiesel quality related to ignition delay time and combustion quality. High values of CN mean better ignition and engine performance properties. The Cetane Number (CN) observed in all the treatments presented a low value to that recommended by the international standards ASTM 6751 and to the minimum by EN14214 (Table 6). However, the high cetane number value and proportions of SFA obtained for all treatments can be associated with efficient combustion properties of biodiesel (Table 6), similar to those reported by other authors [1, 19].
According to Katiyar et al. [28] a high amount of SFAs leads to a high cetane number, indicating complete combustion of biodiesel and smooth engine run, making biodiesel more suitable for vehicles. On the other hand, the formation of pollutants (low emission of NOx) is inversely proportional to the cetane number. The biodiesel generated from S. obliquus where the SFAs dominate the MUFAs has poor cold flow properties; therefore this biodiesel in cold climate regions may be limited, although this problem can be minimized by mixing oil from other species with opposite characteristics [19].
Studies by Wu and Miao [1] reported high CN values (59.30) for S. obliquus compared to the present work of 36.2 and 36.9 in the phototrophic and mixotrophic culture, respectively in urban wastewater (Table 6). The CN values reported by the authors show a positive correlation with the high content of SFA and a negative correlation with DU, suggesting a good ignition of the fuel. Wu and Miao [1] suggest as criteria an SFA / PUFA ratio of 2.31 - 4.02 to obtain a good ignition of biodiesel from S. obliquus.
Comparatively, the SFA / PUFA ratio obtained in the present study was higher (5.02 - 5.94) in the cultures of S. obliquus in urban wastewater (WW), which suggests that the amount of lipids accumulated by microalgae should show a balance between the content of SFA and PUFA, since not always a high value of SFA with respect to PUFA may be the best condition for a good ignition of biodiesel. On the contrary, a high percentage of SFA affects the flow properties causing crystallization / solidification of the fuel in the filters of engine under colder climatic condition.
On the other hand, one of the problems that cause a high proportion of PUFAs is to affect the oxidative stability of the fuel, as can be verified with indicator IV (Iodine Value). This indicator is also a crude measurement of the total unsaturation of biodiesel, which has often been used in connection with issues such as oxidative stability, with the implication that biodiesel with high IV value is less stable to oxidation than that with low IV value [29].
In the present study, the cultures showed IV values lower than 120 g I2 100 g-1 lipids; according to the values established by the European standard, which can be confirmed with the low values of the Degree of Unsaturated (DU) and with a positive correlation between IV and DU; suggesting for all treatments at minimum Oxidative Stability (OS) of 3 to 6 min (Table 5). The IV, DU and OS values compared with other studies indicated for S. obliquus were similar, within the range established as quality criteria by the ASTM D6751 and EN 14214 standards (Table 6), [1, 17-19, 31] indicating that biodiesel obtained from S. obliquus presents a good oxidative stability.
| Biodiesel Properties | Units | ASTM D6751 |
EN 14214 |
[1] | [17] | [18] | [19] | [31] | Phototrophic Culture | Mixotrophic Culture | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AW | WW | AW | WW | |||||||||
| CN | Min | 47 | 51 | 57.93 | 57.13 | 51.74 | 63.63 | 59.98 | 40.2 | 36.9 | 31.5 | 36.2 |
| IV | g I2 /100 g | - | 120 | 72.81 | 77.91 | 98.86 | 35.38 | 77.36 | 27.2 | 41.7 | 67.42 | 45.03 |
| CFPP | °C | - | ≤5/≤-20 | -5.68 | -0.04 | 3.5 | -11.87 | -3.19 | -7.1 | -6.02 | -7.7 | -8.45 |
| SV | mg KOH/g | - | - | 194.8 | 192.4 | - | 216.0 | 164.6 | 315.3 | 325.9 | 245.6 | 313.5 |
| DU | % wt | - | - | 83.97 | - | - | 36.63 | 87.04 | 30.83 | 46.6 | 68.02 | 50.1 |
| OS | h | 3 | 6 | 7.31 | - | - | - | 3.53 | 6.6 | 5.9 | 5.2 | 5.84 |
Another quality criterion analyzed was the Cold Filter Plugging Point (CFPP), a parameter usually used to predict the flow performance of biodiesel at low temperature. Low temperature properties depend mostly on the saturated fatty acids content and the effect of unsaturated fatty acid composition can be considered negligible [30]. The value of CFPP (Value: -6.02 a - 8.45) obtained in the present study suggests that the quality of biodiesel satisfies international criteria with an acceptable limit of -5 °C to 13 °C (Table 6) proposing S. obliquus as a candidate in the production of biodiesel and with potential application in urban wastewater treatment technologies.
The differences found in each of the quality parameters (CN, IV, CFPP, SV, DU and OS) with respect to other reported studies (Table 6) is probably attributed to different factors such as: the composition of the culture medium, light intensity, culture systems (phototrophic, mixotrophic, heterotrophic), nitrogen limitation, lighting periods and the physiological potential of each microalgae strain. It is a fact that the quality of biodiesel depends strongly on the composition of the fatty acids, which can be modified according to the environmental conditions (culture medium) and the physiological potential of each algal strain. The difference in the fatty acids composition can be a reference since the degree of saturation / unsaturation represents an important discriminatory factor to estimate the quality of biodiesel. In accordance with other studies (Table 6), [1, 17-19, 31] it can be concluded that microalgae rich in SFA will generate biodiesel with high CN and low IV and high oxidative stability, while species with a high PUFA content (high DU) are considered to generate biodiesel with low CN and high IV and are more prone to oxidation (Table 6). The physico-chemical properties of Scenedesmus obliquus base biodiesel production compared with international standards (EN and ASTM).
CONCLUSION
The present study shows the potential of the use of a low-cost algal culture medium such as urban wastewater for the production of high-quality biomass and biodiesel compared to a conventional medium. In mixotrophic culture, S. obliquus showed the capacity to increase the lipids productivity over 5% in urban wastewater. The fatty acid profile for S. obliquus reveals a biodiesel used exclusively for urban vehicles since it has a low PUFA content and a high SFAs content. Similarly, high values of CN show a positive correlation with the high SFA content and negative correlation with DU, suggesting a good ignition of the fuel, which is favorable for the emission of pollutants. This proposes S. obliquus as a candidate in the biodiesel production and with potential application in urban wastewater treatment technologies with the possibility of use in phototrophic and mixotrophic cultures; However, the characteristics of wastewater (nutrients, organic carbon, etc.) can have a direct effect on the fatty acids profile and biodiesel quality, which should be considered in future research. In the present study, the effluents of domestic wastewater used in the cultures showed not to have a negative effect on the biodiesel quality.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Authors acknowledgement to the members of CAEC in Environmental Engineering and Universidad Autónoma del Carmen (UNACAR) for encouragement and support.


