All published articles of this journal are available on ScienceDirect.
Comparison of Antibacterial Activity of ZnO Nanoparticles Fabricated by Two Different Methods and Coated on Tetron Fabric
Abstract
Background:
Zinc Oxide Nanoparticles (ZnO NPs) have wide applications in various industries, especially they have been known for their antibacterial effects in polymers and textile fibers. ZnO NPs were produced by two different solutions and milling methods. Different techniques were used in order to select the most effective methods for coating the fabric with ZnO NPs. The microstructures and the composition of the ZnO NPs were investigated using Field Emission Scanning Electron Microscopy (FE-SEM) coupled with Energy Dispersive X-ray Spectroscopy (EDS) and X-ray diffraction analysis (XRD). Additionally, the antibacterial activity of the treated fabric against Staphylococcus aureus and Escherichia coli bacteria was investigated. The overall experimental findings show that the highest inhibitory effect against Staphylococcus aureus in the sample of fabric which covered with ZnO NPs synthesized by the solution method.
Methods:
In the solution method, ZnO NPs were synthesized by dissolving zinc chloride in 1, 2 Ethanediol and mixing with aqueous solution of sodium hydroxide. In milling method, firstly, zinc sulfide nanoparticles were prepared through reaction between zinc acetate and Thioacetamide and then by milling and oxidation the zinc sulfide nanoparticles, ZnO NPs were synthesized. In order to deposition ZnO NPs on the Tetron fabric, it was fully drawn and fixed on a frame. After that, acrylic copolymer resin was added into distilled water and ZnO NPs were added in another beaker to ethanol. The two beakers were then placed in the ultrasonic bath for a certain time. Finally, the fabric was dipped into the beaker containing resin for some moment and then immersed into the beaker containing ZnO NPs. During these processes, both beakers were in the ultrasonic bath. After drawing out the fabric from second beaker, it was dried in air. This procedure was performed for both types of ZnO NPs fabricated by two mentioned methods. Antibacterial activity of ZnO NPs coated on the fabric against two types of bacteria was studied by agar diffusion method.
Results:
XRD patterns of synthesized powders from both methods were identified as ZnO NPs. Sharp diffraction peaks indicate good crystallinity of ZnO NPs. The morphology of the ZnO NPs fabricated by both methods which was analyzed by field emission SEM shows that the ZnO particles synthesized by milling and solution methods are in nano scale at the range of 26 - 29 nm and 9 - 11 nm, respectively. The highest inhibitory effect against Staphylococcus aureus was shown for the fabric which coated by ZnO NPs produced by the solution method. It was seen, the antibacterial activity of ZnO NPs fabricated by solution method was higher than that of milling method.
Conclusion:
ZnO NPs were synthesized by two different methods and the antibacterial activity of Tetron fabric coated with ZnO NPs was studied. Distribution and stability of ZnO NPs on the fabric depend on fabrication method and particle size which means that the smaller particles have more stability and better distribution than larger particles. The particle size and deposited concentration of ZnO NPs were effective on antibacterial activity, so that the smaller particles tend less agglomeration and have more surface area and because of that better antibacterial activity. Overall the results demonstrated a good antibacterial activity against Staphylococcus aureus than Escherichia coli.in the sample of fabric which covered with ZnO NPs synthesized by the solution method.
1. INTRODUCTION
Inorganic nanoparticles recently have been considered as non-viral vectors [1]. Some of the inorganic materials and metal oxides are known as a safe material for humans and animals and they include Zinc Oxide (ZnO), Magnesium Oxide (MgO), Copper Oxide (CuO), Calcium Oxide (CaO), Titanium Oxide (TiO2) and Silver (Ag) [2-5]. Zinc oxide and silver nanoparticles are used for infectious diseases because of their antimicrobial properties and also zinc oxide has been used in the formulation of personal care products [3, 6, 7]. Zinc oxide has more usage in different areas such as semiconductor materials (UV light emitting diodes, laser diodes, solar cells and acoustic devices) [8-10], photo catalysts (due to their high activity, used for degradation of pollutants in water) [11], protecting oil paintings on paper (against dirt, fungal attack, and UV aging) [12]. Moreover, ZnO NPs stability under harsh processing conditions and relatively low toxicity together with the strong antimicrobial properties fortify their application as antimicrobials [2]. Also, many researchers have shown that some nanoparticles, such as ZnO NPs, have selective toxicity to bacteria and only exhibit minimum effect on human cells [13]. Therefore, ZnO NPs can be used in textile industry for adding the additional properties like antibacterial for using in hospital such as healing, hygienic and medical applications [14, 15]. Many attempts to investigate the antimicrobial effects of zinc oxide nanoparticles on the fabric have been performed. Abramova et al. [16] coated cotton fabric by ultrasonic method with ZnO NPs and evaluated its antibacterial effect against Escherichia coli. The result showed the agglomerated particles on cotton fabric with good resistance against Escherichia coli microorganism. Perelshtein et al. [17] covered cotton bandages with ZnO NPs under ultrasound irradiation and evaluated its antibacterial effect against Escherichia coli (Gram negative) and Staphylococcus aureus (Gram positive). Their results showed that the cotton bandages coated with 30nm ZnO NPs exhibited antibacterial effect against both microorganisms. C. Balakumar et al. [18] prepared ZnO NPs by wet chemical method and directly applied on to the 100% cotton woven fabric using pad-dry-cure method. They showed that the finished fabric demonstrated significant antibacterial activity against Staphylococcus aureus in both qualitative and quantitative tests. In this research, ZnO NPs were prepared by two different methods (solution and milling) and were deposited under ultrasonic waves onto the tetron fabric and then their antibacterial properties against two types of bacteria were studied.
2. EXPERIMENTAL PROCEDURE
2.1. Materials
Zinc acetate (purity >99.5%) and Thioacetamide (purity >99.0%) were purchased from Merck company. Also, Zinc chloride (>90%), 1, 2 Ethanediol (>90%), NaOH (>95%) and acrylic copolymer resin (DM5 from Azar chemical company) were prepared for the experiment.
2.2. Synthesis of ZnO NPs by Milling Method
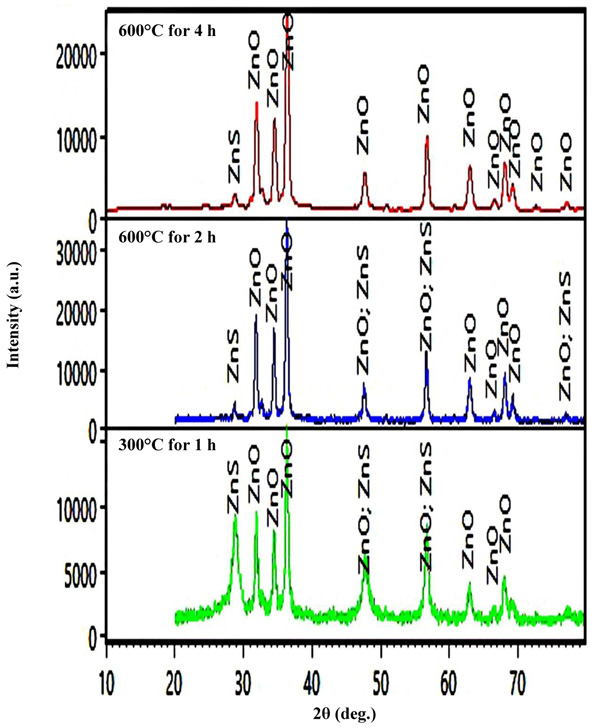
The method includes two steps. In this process, zinc sulfide nanoparticles were produced thereafter zinc oxide nanoparticles synthesized. In the first step, zinc sulfide nanoparticles were prepared through reaction between Zinc Acetate (Zn (CH3COO)2.2H2O) and Thioacetamide (CH3CSNH2). At first, zinc acetate was dried in an oven at 150°C for 30 min. Then, zinc acetate and thioacetamide were milled individually using a planetary ball mill, under the rate of 280 rpm for 20 min. Ball to powder ratio was 20:1. Afterwards, zinc acetate and thioacetamide with the reaction stoichiometric ratio were mixed together (15.64 grams zinc acetate and 5.53 grams thioacetamide) and then milled for 30 min. At the final, the milled powder mixture was heated in an oven at 150°C for 1 hour to achieve zinc sulfide nanoparticles. In the second step, ZnO NPs were synthesized through oxidation of ZnS NPs. For this purpose, ZnS NPs were heated at 600°C in an oven for 4 hours [19].
2.3. Synthesis of ZnO NPs by Solution Method
All chemicals used were purchased from Merck Company (Germany) and were of analytical grade. 11 grams zinc chloride were dissolved in 400 ml of 1, 2 Ethanediol in a beaker. The solution was stirred using a hot plate with magnetic stirrer until zinc chloride totally dissolved in 1, 2 Ethanediol. The temperature of the beaker was kept constant at 150°C. In the meantime, 40 grams sodium hydroxide (NaOH) was dissolved in 200 ml distilled water in a separate container. 32 ml of prepared sodium hydroxide solution was added in the form of drops to the 1, 2 Ethanediol solution under constant stirring. The resulting solution changed into a white colloid without any obvious precipitation. After adding the whole sodium hydroxide, it was given 30 min to do reaction. The solution was allowed to sediment and the supernatant solution was removed by washing 5 times with distilled water. Finally, the sediment was collected by filtering and dried in the air and then changed into powder form [20].
2.4. Deposition of ZnO NPs on the Tetron Fabric
Both types of ZnO NPs which prepared with previous methods were used in this section. The tetron fabric which is made of 65% polyester/35% rayon blend was cut into 8×8 cm2 and it was washed by 2g Sodium Lauryl Sulfate (SLS) in 150 ml distilled water in a beaker to remove the surface pollution. Then, the fabric was fully drawn and fixed on a frame. After that, 5g of acrylic copolymer resin was added into 550 ml distilled water and the same time 0.1g ZnO nanoparticles was added in another beaker containing 500 ml ethanol 96%. The two beakers were then placed in the ultrasonic bath for 10 min. After this time, the fabric was dipped into the beaker containing resin for some moment. Finally, the fabric was immersed into the beaker containing ZnO NPs for 30 min. During these processes both beakers were in the ultrasonic bath. After drawing out the fabric from second beaker it was dried in air. This procedure was done for both types of ZnO NPs fabricated by two mentioned methods.
3. EVALUATION OF ANTIBACTERIAL ACTIVITY
3.1. Preparation of Microbial Strains
Bacterial strains included Staphylococcus aureus ATCC 25923 (PTCC 1431) and Escherichia coli ATCC 25922 (PTCC 1399) which were purchased from the collection of bacteria of Iran.
3.2. Fabric Discs Sterilization
Samples containing antibacterial compounds (ZnO NPs) as well as control sample were cut into discs with a diameter of 10 mm and put them into the glass plates. The plates were then sterilized for 15 minutes at 120° C autoclave.
3.3. Study of Antibacterial Effects
Diffusion method was used to investigate the antibacterial effects of ZnO NPs on fabric samples [21]. At first, one loop of standard strain culture media was cultured on the surface of plates. Then, sterile fabrics discs with a diameter of 10 mm were fixed on the surface of culture medium. After that, the plates were incubated for 24 hours at 37° C. In the disk diffusion method 1.5×108 CFU /ml (equivalent to 0.5 McFarland standards) of standard culture of each strain was cultured on agar surface at the first step, then it was spread on the surface of agar by sterile glass spreader. Antibacterial activity was observed as inhibition zone on Petri plates. Size of the inhibition zone was measured in millimeters using a metric ruler [22, 23].
4. CHARACTERIZATION
Field emission scanning electron microscopy was carried out using a Mira 3-XMU to evaluate structure and morphology of ZnO NPs. X-ray diffraction analysis was carried out using Philips X’pert diffractometer with Cu-kα (λ=1.54 Å) radiation operated at 40 kv and 30 mA to detect impurities and phases. The crystallite sizes of samples were estimated using the scherrer method. The diffraction angle was varied between 10° and 80°. Ultrasonic bath with 28 KHz frequency was used to disperse the ZnO NPs in the solution.
5. RESULTS AND DISCUSSION
5.1. X-ray Diffraction Analysis
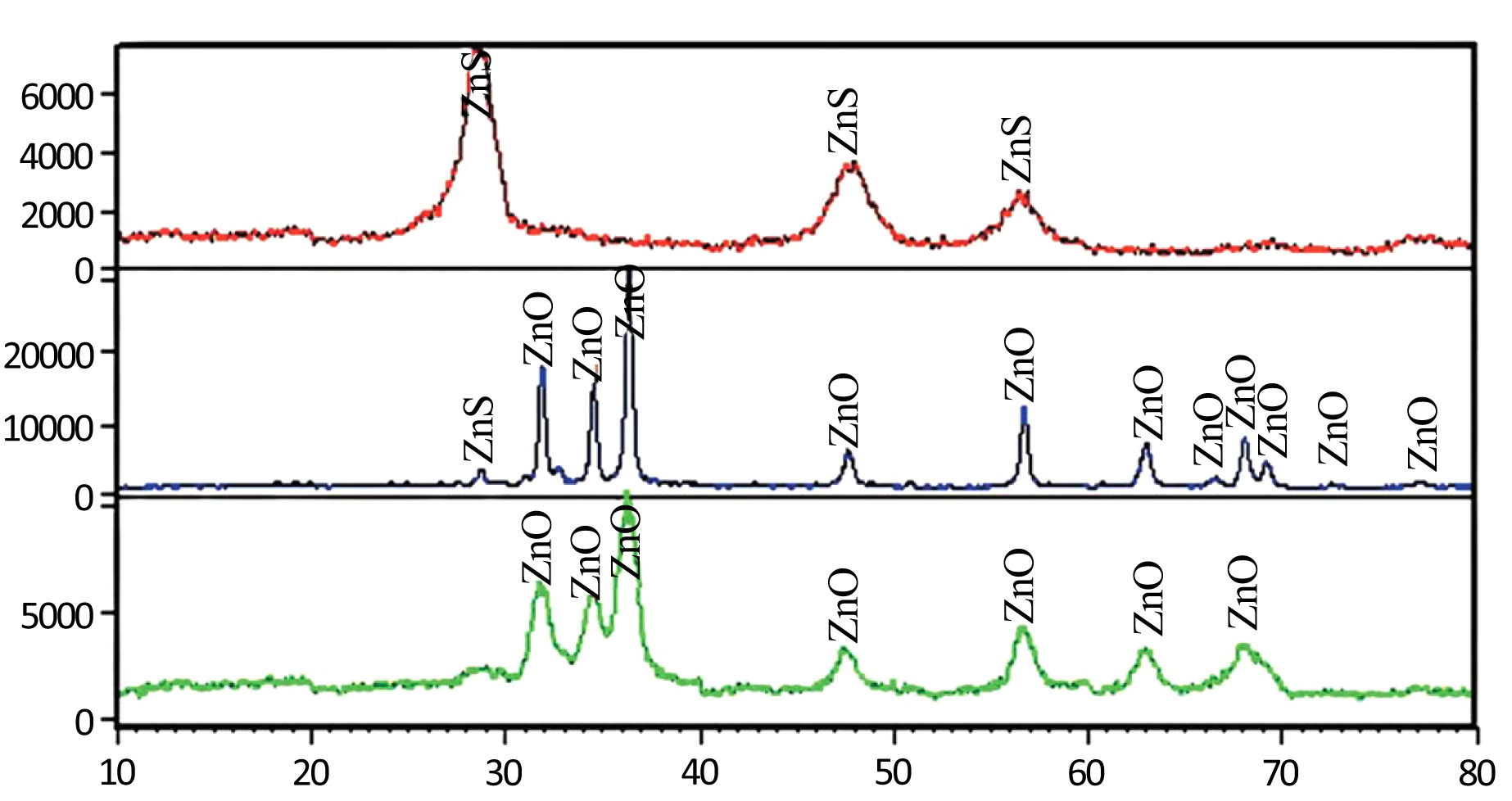
Fig. (1a) shows the X-ray diffraction pattern of the ZnS particles precursor fabricated by milling and heating method. The peaks at 2θ = 28.53, 47.45, 56.30 are appointed to the (111), (220) and (311) reflection lines of Cubic ZnS particles, respectively. Fig. (1b) shows the X-ray diffraction pattern of the synthesized ZnO NPs by milling method. The reflections were indexed according to the diffraction pattern of hexagonal wurtzite-type ZnO. This pattern is in good agreement with reports [19]. The peaks at 2θ = 31.83, 34.48, 36.32, 47.60, 56.66, 62.97, 68.01, 69.09, 72.49, 77.17 are appointed to the (100), (002), (101), (102), (110), (103), and (112) reflection lines of hexagonal ZnO particles, respectively. Sharp diffraction peaks shown in Fig. (1b) indicate good crystallinity of ZnO NPs. The peak with very low height at 2θ = 28.71 is attributed to ZnS phase which indicates that the calcination is not done completely. Grain size of products was calculated using Debye-Scherrer equation:
 |
(1) |
Where D is the average grain size, λ is the wavelength of X-ray which is equal to 1.54Å, θ is the Bragg diffraction angle, and B is the line broadening at half the maximum intensity (FWHM), in radians. Due to the Debye-Scherrer formula, the grain size of nanoparticles was 8 nm.
Fig. (1c) shows the X-ray diffraction pattern of the particles synthesized by the solution method. XRD patterns of powder were identified as ZnO NPs indicating that the solution method also leads to zinc oxide particles. Based on the Debye-Scherrer equation, the grains size is about 2 nm. Fig. (2) shows the different thermal heating regimes for milled samples. It shows that the peaks of ZnS as an impurity decrease with increasing time and temperature. Therefore, the optimum time and temperature were chosen from the heating regimes.
5.2. Field Emission SEM
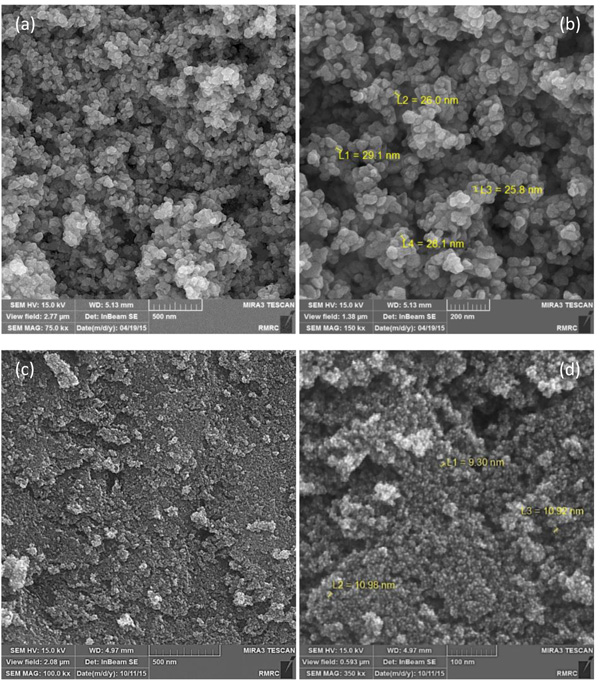
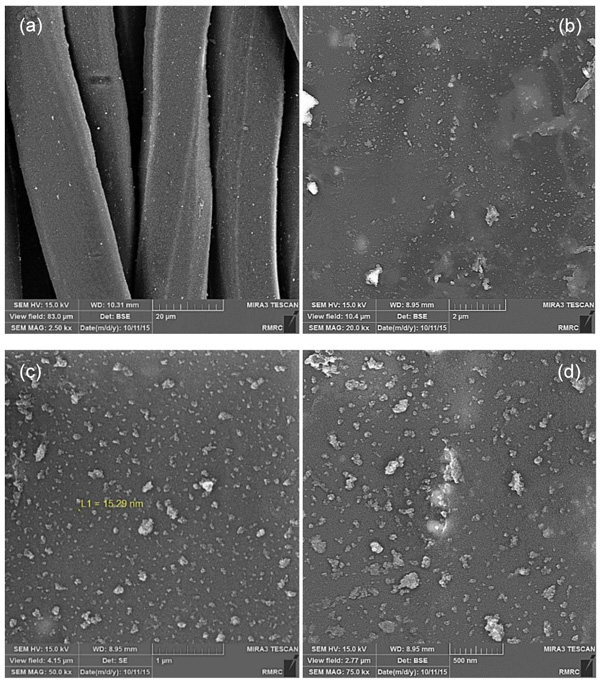
The morphology of the ZnO NPs fabricated by both milling and solution methods which was analyzed by field emission SEM is presented in Fig. (3). Fig. (3a, b) shows the ZnO particles synthesized by milling method which are in nano scale at the range of 26 – 29 nm. Fig. (3c, d) shows the ZnO particles produced by solution method with the particle size of 9 to 11 nm.
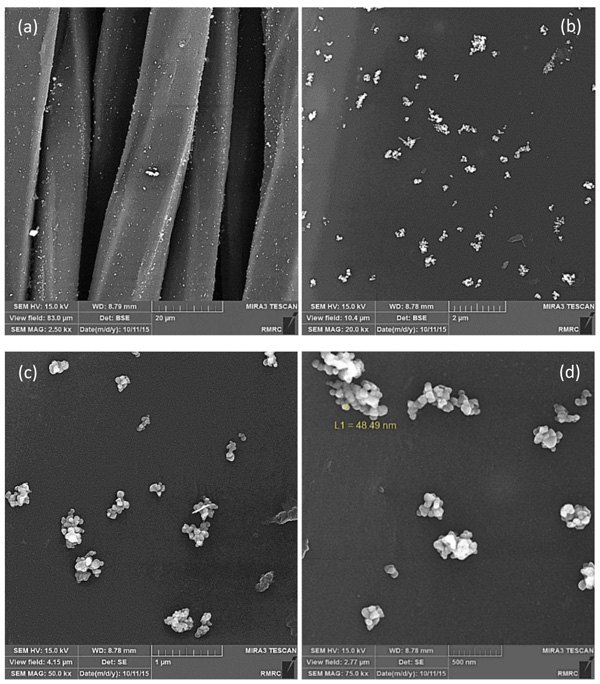
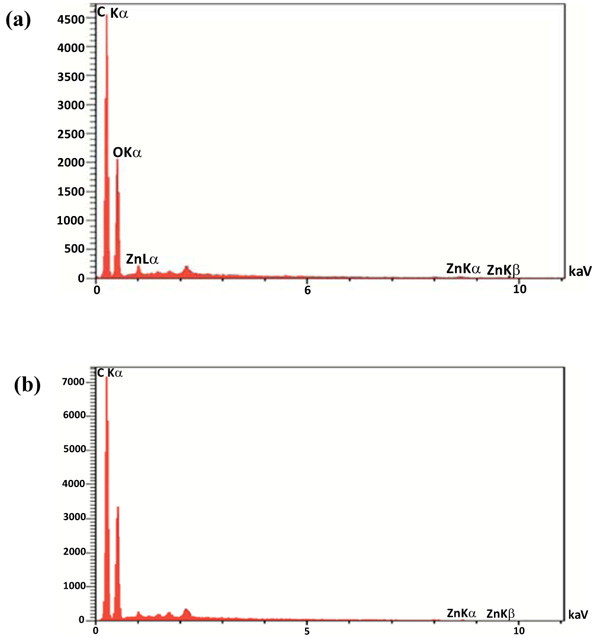
After coating the surface of Tetron fabric with ZnO NPs, the morphology and distribution of the powders were analyzed using a field emission scanning electron microscope. SEM images of the sample coated with ZnO NPs produced by milling and solution methods are presented in Figs. (4 and 5), respectively. The SEM images of the treated fabrics presented Zinc oxide NPs deposited and embedded onto the surface of fabric fibers. Figs. (4 and 5), the top left images, show several fabric fibers coated by ZnO NPs in lower magnification. The scale bar is indicated 20 micrometers which is relatively equal to fibers width. It can also be seen from Figs. (4a to d) and (5a to d), that the distribution of ZnO NPs fabricated by solution method on the surface of the fibers is much better and the agglomeration is much less than that of milling method. From the SEM images the higher aggregation density of ZnO nanoparticles is observed on the fabric fibers in solution method and it causes to achieve better antibacterial activity and stability because of the smaller size and distribution of ZnO NPs in solution method. The large agglomerated particles of ZnO seen in SEM micrographs are probably due to sintering of ZnS powders during calcination treatment. The composition of the nanoparticles on the fabric was proven by quantitative energy dispersive X-ray elemental microanalysis (EDS) shown in Fig. (6).
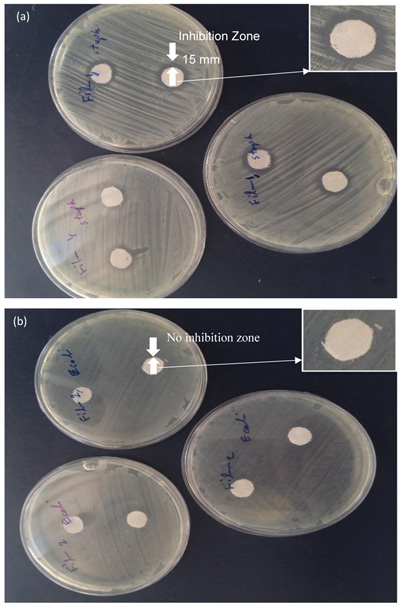
The EDS spectrums show the peaks of Zn, C and O. The presence of C is probably due to the fabric and the peak in 2.7 Kev is appointed to S which is in agreement with the XRD results. The agar diffusion method is a relatively quick and easily executed semi-quantitative test to assess antibacterial activity of diffusible antibacterial agents on treated textile material [21]. The use of zinc oxide nanoparticles due to the reactive oxygen species formed from these nanoparticles, which results in the production of hydrogen peroxide from the surface of zinc oxide can be effectual for the inhibition of bacterial growth and antibacterial activity [24]. Therefore, antibacterial activity of ZnO NPs against two types of bacteria was studied by agar diffusion method (Fig. 7). According to this method, the antibacterial activity of fabrics was shown by the diameter of the zone of inhibition in comparison to the control fabric. The experiment was performed six times and the mean value was taken. Fabric discs (10 mm) with or without ZnO NPs coating were tested and the results are presented in Table 1. As shown in Table 1, the highest inhibitory effect against Staphylococcus aureus is related to the sample 1 which coated by ZnO NPs produced by solution method. The average inhibition zone was 14 mm.
The average inhibition zone diameter of the fabric sample 2 coated by ZnO NPs fabricated by milling method was about 12.2 mm. As a consequence, the antibacterial activity of ZnO NPs fabricated by solution method was higher than that of milling method as well as the small size and high surface-to-volume ratio of nanoparticles allow for better interaction with bacteria [4] On the other hand, the concentration of H2O2 increases with decreasing particle size [25]. This is most likely because of the ZnO particles size which is smaller in sample 1 than that of sample 2 [26, 27]. Although antibacterial activity is observed against Staphylococcus aureus, the fabrics coated with ZnO NPs do not show any inhibition against Escherichia coli. This could be related to the concentration of ZnO NPs on fabric which was not enough to prevent bacterial growth. It should be noted that Gram-negative bacteria such as E. coli are more resistant to antibacterial compounds than Gram-positive bacteria [28].
| Fabric Samples | Microorganisms | Inhibition Zone Diameter (mm) | Average | Standard. Deviation | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| Control | Staphylococcus aureus (PTCC 1431) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Escherichia coli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (PTCC 1399) | |||||||||
| Sample 1 | Staphylococcus aureus (PTCC 1431) | 15 | 15 | 13 | 13 | 14 | 14 | 14 | 0.89 |
| (solution) | Escherichia coli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (PTCC 1399) | |||||||||
| Sample 2 | Staphylococcus aureus (PTCC 1431) | 15 | 12 | 12 | 11 | 11 | 12 | 12.2 | 1.47 |
| (milling) | Escherichia coli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (PTCC 1399) | |||||||||
CONCLUSION
ZnO NPs were synthesized by two different methods and the antibacterial activity of tetron fabric coated with ZnO NPs was studied. Distribution and stability of ZnO NPs on the fabric depend on fabrication method and particle size which means that the smaller particles have more stability and better distribution than larger particles. The particle size and deposited concentration of ZnO NPs were effective on antibacterial activity, so that the smaller particles tend less agglomeration and smaller particles have more surface area and because of that better antibacterial activity. Overall, the results demonstrated a good antibacterial activity against Staphylococcus aureus than Escherichia coli.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


