All published articles of this journal are available on ScienceDirect.
The Geomicrobiology of Chromium (VI) Pollution: Microbial Diversity and its Bioremediation Potential
Abstract
The role and significance of microorganisms in environmental recycling activities marks geomicrobiology one of the essential branches within the environmental biotechnology field. Naturally occurring microbes also play geo-active roles in rocks, leading to biomineralization or biomobilization of minerals and metals. Heavy metals, such as chromium (Cr), are essential micronutrients at very low concentrations, but are very toxic at higher concentrations. Generally, heavy metals are leached to the environment through natural processes or anthropogenic activities such as industrial processes, leading to pollution with serious consequences. The presence of potentially toxic heavy metals, including Cr, in soils does not necessarily result in toxicity because not all forms of metals are toxic. Microbial interaction with Cr by different mechanisms leads to its oxidation or reduction, where its toxicity could be increased or decreased. Chromite contains both Cr(III) and Fe(II) and microbial utilization of Fe(II)- Fe(III) conversion or Cr (III) - Cr (VI) could lead to the break-down of this mineral. Therefore, the extraction of chromium from its mineral as Cr (III) form increases the possibility of its oxidation and conversion to the more toxic form (Cr (VI)), either biologically or geochemically. Cr (VI) is quite toxic to plants, animals and microbes, thus its levels in the environment need to be studied and controlled properly. Several bacterial and fungal isolates showed high tolerance and resistance to toxic Cr species and they also demonstrated transformation to less toxic form Cr (III), and precipitation. The current review highlights toxicity issues associated with Cr species and environmental friendly bioremediation mediated by microorganisms.
INTRODUCTION
Microorganisms play critical geo-active roles in the environment. They are involved in biogeochemical element recycling and bio-transformation of metals and minerals, bioweathering and bioleaching in soils and sediments. The microbial biochemical and metabolic activities have an effect on metal speciation, solubility, mobility, bioavailability, and toxicity [1]. Some of these mechanisms are essential and considered as a part of biogeochemical cycles for metals-recycling, where the metals are involved in microbial metabolism, development, and cell-differentiation [2, 3]. Microbial resistance to toxic metals is common, ranging from limited in uncontaminated environment up to 100% in highly contaminated environment [3, 4]. The survival of these microbes depends on protective mechanisms: redox bio-transformations, expression of metal binding stress proteins, precipitation, transport, efflux and intracellular compartmentalization. Significant metal binding abilities of cell walls and other cell structural components lead to different mobility rates [3, 4]. Chromium belongs to the heavy metals, which are considered as human hazards and thus microorganisms are required to control its concentrations. A slight elevation in the level of Cr (VI) elicits environmental and health problems because of its high toxicity, mutagenicity and carcinogenicity, while the reduced trivalent form (Cr (III)) is less toxic and an essential nutrient for living organisms [5].
CHROMITE
Chromite (FeCr2O4) is a brownish black, weekly magnetic cubic mineral belonging to the spinel group [6]. It mainly forms in ultramafic igneous rocks (peridotite) where it is accumulated during the early stages of magma crystallization [7]. It is also found in serpentinites, which develop through hydrothermal alteration of peridotites. Since its discovery in 1798, chromite is still considered as an economic source for chromium extraction. Theoretically, it is mainly composed of around 32.0% FeO and 68.0% Cr2O3 and, also contains a number of additional elements, such as Al, Ti, Mg and V, which either substitute for Cr or Fe, respectively [6]. Generally, chromite is chemically inert and insoluble in water. The annual production of chromite ore was 23,700-24,000 x103 metric tons, in year 2010-2011. The estimated world reserve is projected at >480 x 106 metric tons of shipping-grade chromite ore with approximately 45% Cr2O3. The foremost resources are situated in Kazakhstan (220 x106 t), southern Africa (200 x106 t), India (54 x106 t) and United States (0.62 x106 t) [8]. Chromium is extensively used in metallurgical and chemical industries for the production of ferrochrome and chemicals, such as sodium dichromate. Chromate production from chromite ore usually leads to high environmental pollution with a low extraction ratio. Usually, the processing of chromite is done by roasting with sodium carbonate at 1200 °C, in a rotary kiln with the addition of limestone and dolomite, ending up with >70% yield of Cr [9].
CHROMIUM CYCLE
Soil composition, texture, physical conditions in the soil, and the flora are the major aspects influencing the Cr mobility [10]. The oxidation states of Cr in aqueous environments are +2, +3 and +6; although +3 and +6 are the most common. Compared to Cr (III), aqueous hexavalent chromium (Cr (VI)) is the most oxidized, mobile, reactive, and toxic form of Cr with no sorption in most sediment at pH> 7 [11]. The hexavalent chromium speciation at different pH values are shown in Table 1. The common Cr (III) species include Cr(OH)3 as aqueous and solid form, while Cr(OH)4- [pH > 9], CrOH2+, Cr(OH)2+, and Cr3(OH)45+ occur in solution.
| Cr (VI) species | pH |
|---|---|
| Monochromate anion (HCrO4-) | < 6.5 |
| Chromate anion (CrO42-) | > 6.5 |
| Bichromate anion (HCr2O7-) | < 1 |
| Dichromate anion (Cr2O72) | 1< pH <7.5 |
| Chromate anion (CrO42-) | > 7.5 |
Under alkaline to slightly acidic conditions, chromate (CrO42-), bichromate (HCrO4-), and dichromate (Cr2O72-) are weakly attached to loams leading to a high mobility in the subsurface [12]. On the other hand, Cr (III) is much less mobile and precipitates readily as Cr(OH)3 or FexCr1-x(OH)3 at pH values >6.0, in soil [12]. Reduction of Cr (VI) to Cr (III) is an effective means of immobilization and can be induced by inorganic or biological agents. The principal chemical reactions leading to chromium cycling are hydrolysis, oxidation-reduction, and precipitation [10]. In partial equilibrium with oxygen - soils and sediments contain Mn-oxides and carbon, which also play an important role in redox reactions with Cr, such reactions are thermodynamically spontaneous [13]. Mn-oxides have high inner surfaces (e.g., tunnels in structure) and possess a high cation exchange capacity, thus acting as strong scavengers for heavy metals under neutral pH condition. Cr (III) oxidation (Eq. 1) in soil is directly proportional to Mn(IV) oxides in the soil [14]. Organic matter, such as hydroquinone has been observed to reduce Cr (VI) in soils as well (Eq. 2) [13].
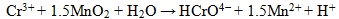 |
(1) |
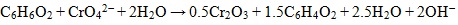 |
(2) |
In general the oxidation of Cr (III) to Cr (VI) in environment is quite difficult as compared to the reduction of Cr (VI) to Cr (III). In general, under normal environmental conditions, a higher pH value favors the oxidation while a lower pH value favors reduction [13].
MICROBIAL DIVERSITY IN CHROMITE
Microbial communities residing in igneous rocks remain inadequately characterized. These rocks constitute approximately 95% of the Earth’s crust. Investigating such microbiological habitat is essential to understand which organisms are involved in the initial weathering of rocks and minerals and the mobilization of heavy metals, such as Cr [15]. Generally, rocks are considered an extreme environment for microbial communities because they are unvegetated and nutrient-limited. Characterizing microbial communities within chromite could yield new insights as found in other extreme endolithic communities [16]. Some researchers reported different culturable and unculturable microbiological methods (molecular biology tools) to characterize the diversity and abundance of microbes occupying in weathered, nutrient-limited terrestrial chromite rocks. Chromium tolerant and reducing bacteria and fungi have been isolated mostly from chromium-contaminated soil, wastewater, and industrial effluents [17]. Kourtev et al. [18], reported the effect of hexavalent chromium concentration on microbial communities using denaturing gradient gel electrophoresis (DGGE), where the addition of chromium led to enrichment and emergence of different microbial populations. Dos Santos et al. [19], tested the microbial diversity using soil microcosms contaminated with crude oil with or without Cr and Cu by performing DGGE of PCR amplified 16S rDNA. The oil contaminated soil showed normal growth with a genetic diversity while the oil and metal contaminated samples had low microbial growth in comparison with the control group/experiments.
CHROMIUM POLLUTION
The CrO42- and HCrO2- ions are two highly mobile forms of Cr in soils, which can be readily absorbed by plants and easily be leached out from decomposing organic matter into different soil layers, thus leading to surface and ground water contamination [20]. In aqueous solution, Cr exists both in trivalent (Cr (III)) and hexavalent (Cr (VI)) forms [21]. Those two forms are commonly dispersed in the environs as a result of different anthropogenic events [22, 23] as well as geochemical mobilization [17]. The industrial processes from which Cr is released include electroplating, petroleum refining, leather tanning, wood preservation, photography, metal finishing, pulp processing, and dye and textile industries [9, 24, 25]. Cr can also naturally be elevated in soils derived from the weathering of Cr-bearing ultramafic rocks and serpentinites which cover approximately one percent of the global land surface [26]. Moreover, mining operations in chromite-sediments often create enormous dumps surrounding the mining area. These waste piles contain waste rocks, unwanted minerals, low-grade chromite ores, and soils. Natural leaching and weathering process occurring in these materials frequently cause hexavalent chromium production and percolation, causing environmental contamination and pollution [8, 27].
CHROMIUM TOXICITY
Chromium (III) is an essential micro-nutrient for plants, animals and humans, which is less toxic than hexavalent chromium [28, 29]. It is reported to play a crucial role in sugar, protein, and lipid metabolism of mammals [8], lowering blood glucose levels to control diabetes, and to reduce blood cholesterol levels. Generally Cr is present in a variety of foods: broccoli, Brewer’s yeast, liver, cheese, whole grain breads, and cereals. Some claims have been made that Cr also facilitates muscle growth [7]. Hexavalent chromium-mediated toxicity is mainly due to its easy uptake across the cell-membrane, which leads to the development of oxidative stress, DNA damage, carcinogenicity, mutagenicity and altered gene expression [30]. Furthermore, Cr (VI) is quite soluble in aqueous environments, in the wide pH range, whereas, Cr (III) tends to be adsorbed on soil surfaces or precipitate at pH values >6.0. Thus, Cr (VI) poses the highest threat as an environmental contaminant, particularly in surface and ground water systems [31]. Cr (VI) is identified as one amongst seventeen highly hazardous chemicals to humans by the United States Environmental Protection Agency (USEPA) [32]. The maximum allowable limit recommended by the World Health Organization for Cr into inland surface water is 0.1 mg/l, and into drinking water is 0.05 mg/l. If ingested in large doses, elevated levels of Cr (VI) may lead to loss of life. The LD50 for oral toxicity in rats ranges from 50 to 100 mg/kg for Cr (VI) and 1900- 3000 mg/ kg for Cr (III) [33]. Higher Cr in soils can potentially cause toxicity to plant life [34, 35]. Due to Cr stress, the reactive oxygen species like H2O2, OH-, and O2 – are produced, which leads to reduction in CO2 fixation, inhibition of electron transport, inactivation of enzymes, and chloroplast disorganization [36-38]. It is somewhat difficult to assess the Cr-toxicity to soil microorganisms, as the environments studied were also frequently contaminated with diverse organic pollutants and heavy metals [39]. It is reported that prokaryotes show higher resistance to Cr (VI) than eukaryotes [40]. In general Gram-positive bacteria are reported to be more chromate tolerant than Gram-negative bacteria [41]. It was also reported that Cr negatively affects the microbial activity in soil, and lead to the accumulation of organic carbon [42]. Speir et al.. [43], also reported that short-term Cr (VI) exposures leads to inhibition of enzymes such as phosphatase and sulphatase, and also decreases the microbial population in soil.
CHEMICAL AND BIOLOGICAL TREATMENT OF CHROMIUM (VI)
Hazardous hexavalent chromium wastes pose significant environmental pollution risks [9]. Due to several industrial and manufacturing activities, annually more than 170,000 t of Cr waste are released into the environment [40, 25]. Therefore, several technologies to remove Cr (VI) from aqueous solutions have been developed [24]. The industrial waste and soil are treated by various physico-chemical methods: electrochemical reduction [44], electrocoagulation [45], precipitation, adsorption [46], ion exchange [47] and membrane separation [48]. However, the initial costs to set up the necessary tools and to manage those methods are quite high for treatments on a bigger scale. In addition, most of these approaches are quite incompetent due to incomplete contaminant removal, higher chemical usage and energy requisites, while still polluting the water tables due to the production of secondary hazardous wastes. The major limitations of some of those methods are - cost-effectiveness only at high or reasonable contaminant-concentrations and not at lower concentrations (1 to 100 mg/l) [49].
Microbe-based technologies can be applied as a cost-effective technique for contaminant-metal removal, especially from sites containing lower contaminant-concentrations [50]. Bioremediation offers huge advantages for the development of detoxification technologies for Cr (VI)-contaminated soils [27, 51]. Microbial transformation of toxic Cr (VI) provides the tools for green technologies which are more cost-effective. Biotechnological approaches used to limit the toxicity of metal pollution are achieved by selectively enriching those naturally occurring microorganisms to treat particular toxic wastes. The processes by which microorganisms influence removal and recovery of the toxic metals are: biosorption (passive uptake of metal without reduction), bioaccumulation (active uptake without reduction), and biotransformation by enzymatic reduction [52, 53]. Chromium (III) is approximately one thousand-times less toxic than chromium (VI), because the cell membrane is almost impermeable to Cr (III) [54]. For this reason, the biotransformation of chromium (VI) to chromium (III) and precipitation of Cr (III) has been considered as a promising way of treatment by various authors [55, 56]. The first reported bacterial strain with the ability to reduce Cr (VI) was a Pseudomonas sp., isolated from industrial wastewater in 1977 [57]. Several researchers have reported different microbes resistant to Cr (VI) isolated from different sites (Table 2). There are different mechanisms that microbes use to overcome the chromium toxicity in their environment, including oxidation-reduction (redox) reactions, sorption-desorption, and precipitation-dissolution [58].
| Microorganism | Source of Isolation | Chromium tolerance/resistance | Reference |
|---|---|---|---|
| Brevundimonas sp., Sphingomonas sp. and, Azospirillum sp. | Magnetite mine drainage from Hebei China | Chromate-resistant and reducing bacteria | Lu et al.. [71], |
| Bacillus sphaericus | Serpentine soils of Andaman, India | Chromate-resistant and reducing bacteria | Pal & Paul [72], |
| Bacillus sp. JDM-2-1 and, Staphylococcus capitis | Wastewater samples, Sheikhupura, Pakistan | Reduced 81-85% of the Cr (VI) to Cr (III) at pH 7, 37 °C after 96 h | Zahoor & Rehman [73], |
| Staphylococcus aureus | Sediments of Lanzhou Reach of the Yellow River, China | Aerobically reduce 94.5% of 0.4 mM Cr (VI) to Cr (III) in 120 h | Zhang et al. [74], |
| Bacillus sp. | Chromate contaminated soil, India | Cr resistant and reducing bacteria (enzyme - chromate reductase) | Elangovan et al. [75], |
| Desulfovibrio vulgaris | Hildenborough strain (DSM 644) | Accumulated precipitates of Cr (III) on their cell surfaces | Goulhen et al. [76], |
| Cellulomonas sp. | Cr(VI) impacted core from a borehole in a dichromate plume on the US Department of Energy’s Hanford facility in southeastern Washington State | Reduction of Cr (VI) to Cr (III) | Viamajala et al. [77], |
| Arthrobacter sp., and a Bacillus sp. | Long-term tannery waste contaminated soil, Mount Barker, South Australia | Cr (VI)-reducing ability and resistance to Cr(VI) | Megharaj et al. [22], |
| Pseudomonas sp. strain RNP4 | Long-term tannery waste contaminated soil, Chennai, India | Cr (VI) reduction | Rajkumar et al. [78], |
| Serratia marcescens | Local tannery effluent, Concepción, Chile | Cr resistant and reducing bacteria (enzyme - chromate reductase) | Campos et al. [79], |
| Acinetobacter and Ochrobactrum | Activated sludge of a wastewater treatment plant, central Portugal | Cr (VI)-reducing ability and resistance to Cr(VI) | Francisco et al. [80], |
| Enterococcus casseliflavus | Tannery effluent, Vellore district, India | Reduce the Cr (VI) through adsorption process | Saranraj et al. [81], |
| Enterococcus gallinarum | Tannery waste-contaminated soil, Fez, Morocco | Reduce chromate to 100% at a concentration of 200 mg/ l, in aerobic conditions | Sayel et al. [82], |
| Pseudomonas sp., | Industrial wastewater | Reduce Cr (VI) | Romanenko & Korenkov [57], |
| Ochrobactrum intermedium BCR400 | Cr contaminated soil collected from Vadodara, Gujarat, India | Reduced 100 mg Cr(VI)/L completely in 52 h with initial Cr(VI) reduction rate of 1.98 mg/L/h. | Kavita & Keharia [83], |
| Bacillus sp. BT1 | Cr polluted soil collected from Tannery industry, Tamilnadu, India | In the presence of anthraquinone-2-sulfonic acid (AQS), it reduced a total of 400 mg Cr(VI)/L | Kavita & Keharia [84], |
| Aspergillus sp. | Cr contaminated sites | Cr(VI)-reducing ability and resistance to Cr(VI) | Coreño-Alonso et al. [85]; Fukuda et al. [86]; Shugaba et al. [34]; Srivastava & Thakur [34], |
| A. flavus, A. niger, Fusarium solani, and Penicillium chrysogenum | Contaminated peri-urban agricultural soils of Faisalabad, Pakistan | Cr tolerant capability | Iram et al. [87], |
| Trichoderma species | Cr contaminated sites | Chromium tolerant and resistant capability | Debpali et al. [88]; El-Kassas et al. [89]; Shriram et al. [90], |
| Paecilomyces sp. | Isolated from Mexico | Complete disappearance of Cr (VI), with the concomitant production of Cr (III) | Cárdenas-González & Acosta-Rodríguez [68], |
| Glomus intraradices (mycorrhizal fungus) | Chromium contaminated agricultural soil, Salt Lake City, Utah | Cr tolerance and hyperaccumulation | Davies et al. [91], |
| A. niger and A. flavus | Contaminated soil and water, Guimaras Province, Philippines | Cr tolerance (600 ppm) | Bennett et al. [92], |
| Aspergillus niger and T. viride | Chromium contaminated soil, Tamilnadu, India | 30.8 - 83.4% reduction | Sunitha & Rajkishore [93], |
| T. harzianum | Provided by Center for advance studies in botany (CAS), University of Madras, India | Removal by biosorption (90.2%) | Sarkar et al. [94], |
| Aspegillus nidulans, Rhizopus arrhizus, T. viride | Samples of sewage, sludge and industrial effluents, Haryana, India | Uptake capacity of 2.55 mg/g | Kumar et al. [95], |
| Rhizopus oryzae | Procured from IMTECH, Chandigarh, India | Cr reduction efficiency of 91.15% | Sukumar [96] |
Bacterial Reduction of Chromium (VI)
Even though heavy metals are quite toxic to majority of microorganisms, some bacteria developed the resistance mechanism. Several habitats exposed to anthropogenic or natural metal contamination over an extended period of time showed that long-term exposure to those metals acted as a selective-enrichment which favored the growth of metal-tolerant microbes [59]. The transformation of Cr (VI) to Cr (III) in the microbial cells leads to the formation of intermediate 'O' radicals and unstable oxidation states of chromium respectively (such as Cr(V) and Cr(IV)), which are more toxic than Cr (III) [60]. Despite these highly reactive compounds such unique microorganisms have alternative mechanisms to overcome these problems: chromate resistant plasmids, iron efflux systems, and variations in the reduction mechanisms [22, 60, 61]. Generally, chromium reduction mechanisms can be enzymatic or chemical, where the microbes follow either single or combined process. Aerobic bacteria such as Bacillus, Pseudomonas, Streptomyces, and Leucobacter spp. are associated with soluble chromate reductases (using NADH or NADPH as cofactors to reduce the Cr), whereas the anaerobic ones including Shewanella, Enterobacter, and Sulfate reducing bacteria (SRB) have membrane-bound reductase such as flavin reductase, cytochrome, and hydrogenase, which are part of the electron transport system. They use Cr (VI) as a terminal electron acceptor instead of NO3- or SO42-, as has been reported for Desulfotomaculum reducens [62]. These two Cr (VI)-reducing activities were found in Desulfovibrio vulgaris where Cytochrome c3 was reported to catalyze Cr (VI) and uranium (VI) reduction. Here, the cytochrome may function as both U (VI) and Cr (VI) reductase [55]. However, microbial reduction of Cr (VI) may also take place through chemical reactions associated with intra/extra cellular compounds, such as amino acids, vitamins (vitamin C, in particular), nucleotides, sugars, organic acids or glutathione. In addition, some microbes have their own plasmid resistant mechanism, which is considered to be a powerful detoxification process of Cr (VI) [63].
Bacterial Oxidation of Chromium (III)
It is well-known that chromite has very low water solubility and high chemical resistance to dissolution. The oxidation of Cr (III) could occur via O2-, H2O2, and Mn-oxides. In subsurface environments, the direct oxidation of Cr by oxygen is limited because of the slow kinetics [64]. However, the oxidation of Cr by H2O2 is probably insignificant due to its limited subsurface production. Mn(IV) oxides are the only known naturally occurring oxidants for Cr (III). Karen et al. [65], reported Mn(II) oxidizing Bacillus sp. strain SG-1, which accelerated the Cr (III) oxidation 7-times faster than without bacteria, in the presence of Mn. Mn(II) oxidizing bacteria accelerate the production of reactive Mn(IV) oxides, which are required in the oxidation of Cr (III). Another study conducted with soil containing Mn oxides (Mn3+, Mn4+) showed that the oxidation of Cr (III) took place under aerobic system, in presence of Mn oxidizing Pseudomonas putida species [66].
Fungal Chromium Resistance
Chromium resistance has been reported in both yeast and filamentous fungi isolated from Cr contaminated environments (Table 2). Cr resistance in the yeasts strains including Candida and Rhodosporidium was related to their ability to reduce ion uptake, rather than to their biological reduction of Cr (VI) to Cr (III). Species like zygomycetes fungus Mucor rouxii and the yeasts Candida albicans, Yarrowia lipolytica, and S. cervesiae were very sensitive to chromium without any change in chromium concentration in the media [55]. In contrast, strain RR1 of Candida sp. showed a high capacity to resist Cr, being highly efficient in reducing Cr (VI) to the less toxic form via contact cells and crude cell-free extracts [67]. Moreover, Paecilomyces sp. showed its capacity to reduce 50 mg/l Cr (VI), with the associated production of Cr (III) in the growth medium after 7 days of incubation [68]. Congeevaram et al. [69], reported that Aspergillus sp. isolated from contaminated site showed a high resistance to chromium of up to 10,000 mg/l via biosorption processes. Viti et al. [70] reported in-depth comparative analysis of molecular mechanisms for Cr(VI) resistance in bacteria and fungi compared with classical microbiological approaches: Both bacteria and fungi respond to presence of Cr(VI) by combining cellular networks systems at several levels, such as the reducing power generated by iron and sulfur metabolism, protein oxidative stress protection, DNA repair, Cr-efflux pumps, and detoxification enzymes - such as Cr(VI) reductases. Chromate-resistance determinants (CRDs) have been reported from different microbes, which consist of genes belonging to the chromate ion transport (CHR) superfamily. Generally, CRDs include the chrA gene, which encodes a putative chromate efflux protein. However it has been reported that chrA genes provide Cr(VI) protection only in very low concentrations range [70]. They concluded that the currently available knowledge is largely theoretical and still a molecular understanding of many aspects of Cr(VI) resistance in microorganisms are poorly understood.
CONCLUSION
Microorganisms exist in different environments and many of those environments have been investigated. Although considerable knowledge about microbial diversity has been amassed, most of these organisms remain uncharacterized, due to several reasons including habitats that have not been investigated, organisms that are difficult to culture in laboratory conditions, and inaccurate identification of compiled samples. Many microbes isolated from the contaminated area were capable of reducing the chromium toxicity and the search continues to find more tolerant microbes. The presence of high chromium-tolerant or/and resistant microbes shows that they have evolved different mechanisms to reduce the detrimental effects on their cells. This brief review based on published literature summarizes the importance of employing indigenous microorganisms to reduce chromium toxicity as a cost effective and environmentally sustainable technology.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


