All published articles of this journal are available on ScienceDirect.
Adverse Effects of Bisphenol A Exposure on Glucose Metabolism Regulation
Abstract
Bisphenol A (BPA) is used as basic chemical compound in the production of polycarbonate food containers or epoxy resins coating metallic cans for food and beverages conservation. Its xeno-estrogenic activity alters endocrine-metabolic pathways modulating glucose metabolism and increasing the risk of developing diabetes, insulin resistance, and obesity. Based on in vitro and in vivo experimental research, here we report some of the major BPA adverse effects on tissues that play a key role in the regulation on the whole body’s metabolism. Evidences have shown that BPA is able to exert its endocrine disrupting action altering glucose metabolism and contributing to the onset of metabolic disorders, acting on liver functions and affecting insulin production by the pancreas. Exposure to BPA has been reported also to modulate glucose utilization in muscles, as well as to interfere with adipose tissue endocrine function. In addition, to peripheral tissues, recent studies have shown that BPA by acting in the Central Nervous System affects neuroendocrine regulation of glucose metabolism, promoting glucose metabolism dysfunction such as glucose intolerance and insulin resistance. Thus, exposure to BPA seems to be an important risk factor in the onset of obesity and metabolic syndrome. However, its mechanisms of action need to be further investigated to provide a major evaluation of risk assessment.
INTRODUCTION
The “Endocrine Disrupting Chemicals” (EDCs) theory proposes that endocrine disruptors are specific chemicals that mimic endogenous hormones and/or interfere with their pharmacokinetics. Many natural and synthetic chemicals have been reported to disrupt the normal function of the endocrine system [1].
The classical definition reported by the Environmental Protection Agency of the United States (EPA-USA) is "An endocrine disruptor is an exogenous substance that causes adverse health effects in an intact organism, or its progeny, secondary to changes in endocrine function" [2].
Epidemiological studies suggest a strong correlation between exposure to specific groups of EDCs and alterations of reproductive system, infertility, increased risk of seminomas, endometriosis and malformations [3]. EDCs exposure is correlated also to an increased number of early abortions associated with occupational exposure to pesticides, long-term effects on reproductive and thyroid functions. The damage induced in utero or in childhood is also responsible for metabolic diseases related to the altered homeostasis of estrogens and androgens [4]. Embryo-fetal exposure is far more dangerous and more difficult to diagnose, since the defects occur after decades. Hormones play an important role at various stages of both embryo-fetal development and organogenesis, thus suggesting that any disruption of hormonal function can affect the correct organism development [5].
Many suspected endocrine disruptors are extensively used and have economical relevance. In particular, the most widely used EDC is Bisphenol A that is reported to cause metabolic dysfunction such as obesity, metabolic syndrome and type 2 diabetes [6].
In this review we discuss findings that correlate exposure to BPA to the onset of metabolic disorders affecting glucose metabolism regulation.
Bisphenol A (BPA), 2,2-bis(4-Hydroxyphenyl) propane, a widely industrial used chemical, has been recently recognized as endocrine disruptor. Its production is about 2-3 million tons per year and it is one of main monomers used in polymerization reactions for polycarbonate plastic production. BPA, for its hardness and resistance, is used for the production of epoxy and polycarbonate resins used for food containers, as feeding bottles for children and plastic bottles, or as epoxy resins coating metallic cans containing food and beverage. Small amounts of BPA can be transferred from the polymeric containers or from the epoxy resins lining the metallic cans to the stored food and water, especially when exposed to high temperature (as during sterilization cycles). For these reasons the main exposure for humans to BPA occurs through the diet with the intake of contaminated food and polluted drinking water [7, 8].
Daily BPA intake through the diet is higher in newborn and children compared to adults. Recent estimates on daily BPA intake based on BPA urine levels at different ages showed that exposure levels during 0-6 months of age are between 0.2 and 11 μg/kg body weight/die, while during 6-12 months is between 1.65-13 μg/kg body weight/die. In 1.5 – 6 years old children urinary BPA levels are estimated to be 0.043-14.7 μg/kg body weight/die, significantly higher than the adults (0.008-1.5 μg/kg body weight/die). Exposed workers showed BPA levels between 0.043 μg/kg body weight/die and 100 μg/kg body weight/die. It has been hypothesized that in utero or post-natal BPA exposure can cause morphological and functional alterations affecting growth and reproduction [9-12]. United States Environmental Protection Agency established 50 μg/kg body weight/die of BPA as reference dose, estimated in function of the Lowest Observed Adverse Effect Level (LOAEL) [13]. This exposure level is considered safe but there are some evidences, further described in this review, that this concentration has some adverse effects in animal models. For this reason, further investigations are needed to be properly understood if the reference dose is adequate for humans.
Many studies have been performed to understand the mechanism of action of BPA as endocrine disruptor and its effects. It was demonstrated that BPA induces estrogen regulated genes and promotes human breast carcinoma MCF-7 tumor cell line proliferation, but with higher concentration than that of estradiol [14]. In vivo mice models confirmed that BPA exerts its estrogenic effects towards classical nuclear receptors, estrogen receptor α and β (ERα and ERβ) but also interacting with several proteins and receptors related proteins as “nonclassical membrane estrogen receptor”, ncmER [15, 16], estrogen-related receptor gamma, ERR-γ [17, 18], G protein-coupled receptor 30, GPR30 [19] and aryl hydrocarbon receptor, AhR [20]. BPA concentration that can mimic the real environmental exposure in humans (2.4 µg/kg body weight/die) is also associated to neural and behavioral alterations; to pancreas, prostate and mammalian gland injuries; to abnormal development of urinary tract, and early puberty in female rodents [21]. Alonso-Magdalena et al. [22] demonstrated that BPA concentration lower than 50 μg/kg body weight/die, correspondent to Tolerance Daily Intake (TDI) established by Scientific Committee on Food [10], is associated with the onset of insulin resistance, characteristic of type 2 diabetes mellitus. Epidemiological studies in humans confirmed that exposure to BPA during critical periods of development can predispose children to obesity and related risk factors such as metabolic syndrome and insulin resistance [23-27], thus indicating that BPA may affect and interfere with glucose metabolism.
BISPHENOL A IMPAIRS GLUCOSE HOMEOSTASIS IN ORGANS CONTROLLING METABOLISM
The obesogenic and/or diabetogenic effects of BPA have attracted the attention of the scientific community since diets and sedentary lifestyles are not enough to explain the great incidence of obesity. Environmental EDCs can affect glucose homeostasis acting on neuroendocrine, pancreatic, liver and adipocyte metabolism [28, 29]. Here we described recent findings on the effect of BPA on glucose metabolism regulation in organs controlling the whole glucose homeostasis, such as liver, pancreas, skeletal muscles, adipose tissue and central nervous system.
BPA Effects in Liver Functions
Liver plays a key role in the regulation of glucose metabolism via hepatic glucose production. In addition, liver is the main organ responsible for the detoxification of the whole body being the center of the metabolism of toxic substances, drugs, xenobiotics and environmental hormones. BPA, belonging to environmental xenobiotic contaminants, reaches the liver where it gets metabolized. Jayashree et al. [30] have investigated the effects in vivo of orally administered BPA in adult male albino rat, on hepatic insulin signaling and the glucose oxidation in liver. They studied the ability of the liver to uptake glucose, to incorporate it into glycogen or oxidate into CO2. They have found that in BPA treated animals circulating insulin levels were increased while testosterone levels were decreased even if fasting blood glucose levels were unaltered compared to that of non-treated rats. Moreover, they described a reduction in hepatic glucose oxidation together with a reduction in the expression of insulin receptors, protein kinase B (Akt), phospho-Akt, and a reduction in glycogen production. Thus, BPA may impair hepatic glucose oxidation increasing glycolysis and promoting the decrease of glycogen synthesis through negative effects on insulin signal transduction with a defect in Akt phosphorylation. BPA has been also described to induce liver damage in growing male rats [31]. Subchronic exposure to BPA induces functional and structural changes such as a significantly increased frequency of edema and parenchymal degeneration in the liver [31]. Naville et al. [32] recently described that some chemical food contaminants, including BPA, at low dose may induce hepatic metabolic dysfunction in the progeny of obese mice. Specifically, this study showed that male mice fed on high fat diet and exposed to the pollutants had an increased peroxisome proliferator-activated receptors γ (PPARγ) and liver X receptor α (LXRα) mRNA levels in the liver. These, in turn, regulate target genes related to lipid and cholesterol metabolism, such as fat uptake and transport cluster of differentiation 36 (Cd36), acetyl-CoA carboxylase 1 (Acaca), sterol response element binding protein 2 (SrebF2), 3-hydroxy-3-methyl-glutaryl-CoA reductase (Hmgcr). As consequence of genes dysregulation, a reduction in hepatic free cholesterol and cholesteryl ester levels was observed together with a decrease of the total cholesterol levels. On the other hand, females displayed a marked glucose intolerance probably due to the increased estrogen sulfotransferase and reduced expression of estrogen receptor.
A recent study by Marmugi and collaborators [33] investigated gene expression changes in the liver of adult male CD1 mice after long-term exposure to five different BPA doses below or equivalent to the No Observed Adverse Effect Level (NOAEL: 5000 mg/kg/day). Specifically, they analyzed the mRNA levels of key genes involved in hepatic carbohydrate metabolism showing that glucokinase (Gck) and pyruvate kinase (L-Pk), involved in glycolysis, were increased in animals which exposed to 500 μg/kg/day of BPA compared to control animals. However, L-Pk expression showed increased mRNA levels already at the lowest BPA dose used in the study (5 μg/kg/day). Furthermore, an overexpression of genes involved in cholesterol biosynthesis, such as 3-hydroxy-3-methyl-glutaryl-CoA reductase (Hmgcr), Mevalonate (diphospho) decarboxylase (Mvd) and Squalene epoxidase (Sqle), were also observed after exposure to BPA doses ranging from 50 to 5000 μg/kg/day. They concluded that BPA, via alteration of hepatic gene expression levels, leads to glucose intolerance, hyperglycaemia and hypercholesterolemia and that these alterations occur also at “safe” exposure doses.
BPA Regulates Insulin Production in Pancreas
Pancreas plays a fundamental role in the regulation of glucose homeostasis via the production and release of two major hormones: insulin and glucagon [34]. While insulin is produced and released by the β-cells, glucagon is produced and released by the α-cells of the pancreatic islets. Defects in pancreatic function can lead to dysregulation of insulin and glucagon production that, in turn, affect glucose homeostasis causing type 2 diabetes and insulin resistance both involved in obesity and metabolic syndrome [35]. It is well known that estrogens are able to regulate pancreatic function, through estrogen receptors expressed in pancreatic cells (mainly Estrogen Receptor α, ERα), with beneficial effects for maintaining insulin sensitivity and β-cells function. Low estrogen levels are reported to be associated with glucose intolerance and insulin resistance [34]. Bisphenol A, being a xenoestrogen compound, was found to mimic estrogen action in pancreas that resulted very sensitive to this chemical. Specifically, it has been reported that BPA induces, via ERα, insulin over-production in the same manner of 17β-estradiol, probably activating extracellular signal-regulated kinases (ERK1/2) pathway [22, 36]. In agreement with these data, Song et al. [37] demonstrated that perinatal BPA exposure in rats induced hyperglycemia thus contributing to insulin resistance of male rats later in life. Similarly, it has been recently described [38] that exposure to BPA during pregnancy disrupts glucose homeostasis in adult male mice resembling the metabolic phenotype of mice fed on a high fat diet. In particular, BPA exposed animals showed fasting hyperglycemia, hyperinsulinemia and glucose intolerance. Male offspring from BPA-treated mothers developed in later life a diabetic phenotype that was associated with obesity. This phenotype was similar to that developed by high fat diet-fed animals not exposed to BPA.
On the other hand, recently, Lin et al. [39] showed the opposite effect of BPA on insulin production using rat insulinoma (INS-1) cells as β-cells model. They showed that exposure to BPA induced apoptosis in INS-1 cells leading to a reduction in cell viability triggered by mitochondria defects, and a decrease in glucose-stimulated insulin production and secretion. Possible explanations to these opposite data are the different models used in the study, i.e.in vivo versus in vitro studies and the doses of BPA used.
Unpublished data from our group are in agreement with these latter findings. We have found that in vivo BPA exposure to mice decreases circulating insulin levels, thus affecting glucose tolerance. However, more experiments are in need to elucidate the mechanisms by which BPA affects circulating insulin levels.
BPA Affects Glucose Utilization in Muscles
Maintenance of glucose homeostasis is a key process providing the correct functioning of the whole body metabolism from the birth to later in life. As already mentioned, this is a complexed process carried out by balancing glucose production and glucose utilization by several tissues involved in metabolism regulation. Skeletal muscles are important in controlling systemic glucose metabolism since tissue glucose uptake induced by insulin mainly occurs in the skeletal muscle. Glucose uptake and utilization take place through glucose transporters, such as glucose transporter-4 (GLUT4) via the activation of intracellular signals that involve insulin receptor substrate (IRS) proteins and Akt signaling [40]. Insulin, in order to control blood glucose levels, promotes glucose utilization in adipocytes and muscles and inhibits hepatic glucose production [41]. To test the involvement of BPA in these processes, Alonso-Magdalena et al. investigated the effects of BPA exposure on glucose metabolism in pregnant mice and in their adult male offspring [42]. They reported an impaired insulin and glucose tolerance together with a reduced activation of Akt signaling pathway in the gastrocnemius muscle both in BPA-treated pregnant mice and male offspring, thus suggesting a reduced peripheral insulin sensitivity. In addition to this study, Indumathi and collaborators [43] found that BPA exposure to adult male rats induced downregulation of insulin receptors, Akt and Akt phosphorylation and the glucose transporter GLUT-4 protein expression both in membrane and cytosol. These data worked together with a reduction in glucose oxidation in the gastroecnemius muscle and an increase in circulating insulin levels, contributed to the development of insulin resistance in these animals.
BPA Affects White Adipose Tissue Physiology and Its Endocrine Activity
The white adipose tissue is an endocrine tissue that produces hormonal signals critical in metabolism regulation. Adipocytes, indeed, produce and release hormones, such as leptin and adiponectin, important endocrine signals in the control of energy metabolism via their actions in target organs including liver, pancreas, muscles and brain [44]. While leptin affects body weight acting mainly on the Central Nervous System and specifically the hypothalamus [45], adiponectin plays a key role in regulating glucose uptake by the cells [46]. Several hormones and growth factors, such as growth hormone, thyroid hormone, insulin, estradiol and also several chemicals with xeno-estrogenic activity, modulate adipocyte development and activity [24, 47-49]. Thus, BPA exposure may have a critical role in interfering with the endocrine adipose tissue function. Specifically, it has been shown that exposure to low BPA doses during the perinatal period results in an increase in body weight in rodents [6], and that BPA effect on white adipose tissue is to induce tissue differentiation. Indeed, in vivo studies showed that BPA modulates the expression of several genes involved in adipocyte differentiation and subsequent function, thus promoting an obese phenotype in rodents. In rats, developmental exposure to BPA resulted in up-regulation of peroxisome proliferator-activated receptor (PPAR), CCAAT-enhancer-binding proteins (C/EBP) transcriptor factors and lipoprotein lipase (LPL) in abdominal adipocytes in adulthood. In mouse embryonic fibroblast cell line 3T3-L1 BPA was found to increase LPL activity and triacylglycerol accumulation interacting synergistically with insulin to further accelerate these processes. BPA was also found to stimulate an increase in the glucose transporter GLUT4 and glucose adipocytes uptake [50-52].
Recently Song et al. [37] investigated BPA effects on glucose metabolism in early and later life of male rat offspring. They found that, after perinatal exposure to BPA, a reduction in adiponectin gene expression and production was observed in male offspring later in life and that at high BPA doses the metabolic dysfunction was detected at earlier age. Interestingly, a recent in vitro study using human adipocytes [53] revealed that low doses of BPA impair insulin-stimulated glucose utilization and insulin signaling pathway thus dysregulating adipocytes function. Furthermore, Angle and collaborators [54], investigating the effects of fetal BPA exposure in male mice, found an increase in body weight and abdominal fat mass, corresponding to an increase in adipocyte number and size. Moreover they found in male offspring higher circulating leptin and insulin levels with a concomitant reduction of serum adiponectin, thus indicating a clear evidence of metabolic dysfunction in white adipose tissue caused by BPA exposure.
BPA Modulates Neuroendocrine Functions Involved in Glucose Metabolism
In the last few years, great attention has been paid on the effects of BPA exposure in the development and function of the Central Nervous System since it has been observed that BPA can accumulate in several areas of the brain. We recently reported in an in vivo study on Balb-C offspring mice exposed to different doses of BPA, an accumulation in the brain of this endocrine disruptor that was dose and gender-dependent [55]. BPA has been reported to affect 1) sexual differentiation in the brain of rodents, 2) cortical development in mice, 3) synaptogenesis in mokeys and rats hippocampus, 4) in prefrontal cortex and midbrain dopamine neurons and 5) hippocampal spine synapses [56, 57].
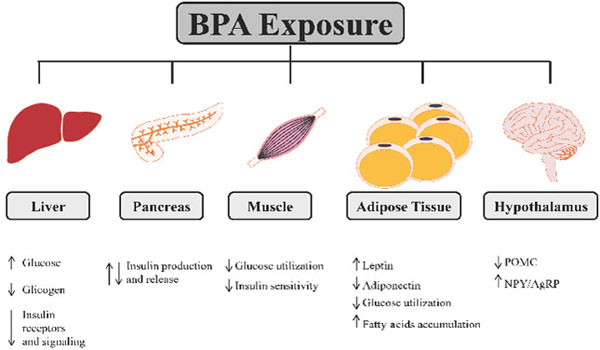
BPA exposure may lead to metabolic dysfunction affecting glucose homeostasis at different levels involving the main tissues that control glucose homeostasis. BPA increases glucose production and reduces glycogen synthesis in liver with a reduction of glucose oxidation and impairment in insulin signaling. In pancreas BPA modulates positively or negatively insulin production and release. In muscles BPA is able to decrease glucose utilization and insulin sensitivity. White adipose tissue endocrine function is modulated by BPA affecting leptin and adiponectin secretion, reducing glucose utilization and increasing fatty acids accumulation. Hypothalamus can be affected by BPA disruption regulating POMC and NPY/AgRP expression in the arcuate nucleus.
The Central Nervous System, mainly via the hypothalamus, regulates feeding behavior and energy expenditure. These functions are, in turn, influenced by peripheral signals, including leptin, insulin, glucose, glucocorticoids, steroids and thyroid hormones. Within the hypothalamus, the melanocortin system, located in the hypothalamic arcuate nucleus, is the main player in appetite and energy homeostasis control. In this system, the pro-opiomelanocortin (POMC) neurons promote satiety associated with glucose utilization by peripheral tissues and suppression of gluconeogenesis by the liver, and the Neuropeptide Y/Agouti related peptide (NPY/AgRP) neurons, activated during fasting or caloric restriction, promote food intake, fat utilization and gluconeogenesis in the liver. Dysfunctions of this system are associated with metabolic disorders, such as obesity and type 2 diabetes [58]. Since it has been demonstrated that a cross-talk between estrogen and leptin signaling in the hypothalamus occurs regulating feeding [45], it is plausible that BPA may be able to alter the central regulation of glucose homeostasis. Few evidences on the effect of BPA on POMC and NPY/AgRP neurons functions have been reported, including those carried out by MacKay and collaborators [59]. In this study, early-life BPA exposure resulted in a more pronounced obese phenotype of the mice exposed to high fat diet. In male mice exposed to high fat diet, early-life BPA exposure lead to a reduction of POMC fiber innervation into the paraventricular nucleus of the hypothalamus and an increase of NPY/AgRP peptides expression in the arcuate nucleus, thus suggesting an increased orexigenic tone. However, female mice exposed to high fat diet showed a decreased POMC gene expression in arcuate nucleus when exposed to high BPA doses. In both genders, early-life BPA exposure induced an impairment in glucose tolerance. Further studies are in need to better understand the mechanisms by which BPA affects metabolism.
SUMMARY AND CONCLUSION
The dramatic increase in the incidence of human obesity and metabolic syndrome has occurred in the last few decades. Since the obesity epidemic cannot be explained only with modification in lifestyle such as sedentary and nutritional habits, the role of environmental chemicals exposure by the diet and their association to metabolic disorders are aspects that need to be considered and studied. Plastics used as food containers are produced using Bisphenol A, an endocrine disruptor that has been recently considered to be involved in the pathogenesis of metabolic disorders including obesity and diabetes. Several studies have shown the ability of BPA to interact and interfere with the functions of many organs involved in the regulation of metabolism.
In vitro and in vivo experiments have shown the adverse effects of BPA on glycogen synthesis, gluconeogenesis, glucose oxidation and insulin signaling pathway in several tissues, including pancreas, liver, muscle, white adipose tissue and Central Nervous System (Fig. 1).
Considering the critical role of these tissues in regulating energy and glucose metabolism and the ability of BPA to affect their functions, it is conceivable that BPA may contribute to the pathogenesis of metabolic disorders. Further investigations are needed to understand the clinical consequences of BPA exposure and the mechanisms by which BPA acts as endocrine disruptor. A better understanding of the outcomes of BPA exposure will allow a more precise evaluation of the risk assessment and will shed a light on the effect of environmental contaminants in the development of metabolic disorders.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
We thank the Interuniversity Consortium INBB for supporting Dr. Ciro Menale with a fellowship.


