All published articles of this journal are available on ScienceDirect.
Evaluation of Oil Quantities in Oleaginous Filamentous Fungi in UAE Wetlands: Potential Precursors of Next-Generation Biofuel
Abstract
Background:
Several studies have suggested that the next-generation biofuels could be produced from the lipids stored by oleaginous fungi. In these microorganisms, lipids are stored as triglycerides (biofuel precursors) converted to fatty acid mono alkyl esters. Fungal growth is very fast and is not impacted by seasonal, climate, and space variations as opposed to plants and animals. Fungi from mangrove ecosystems have not significantly been studied for oil production despite being the second largest group of marine fungi.
Methods:
In the present study, we have analyzed soil samples from mangrove wetlands in Ras Al Khaimah, United Arab Emirates (UAE), for the presence of oleaginous fungal species. We have further characterized the isolated fungi through visual identification and assessed their oil content by gravimetric analysis. In addition, lipid accumulation was examined under fluorescent microscopy.
Results:
A. flavus was estimated to accumulate 25.21% (w/w) while A. niger accumulated 24.34% (w/w) of their dried biomass as lipids.
Conclusion:
The percentages of oil content of the filamentous fungi, A. flavus, and A. niger indicate that these microorganisms are promising sources of next-generation biofuels.
1. INTRODUCTION
Biofuel refers to the fuel derived from biomass processing of carcasses and metabolic byproducts of living organisms, plant residues, and domestic waste. Growing evidence points to the potential of microorganisms such as fungi and microalgae as a viable source of biofuel [1]. Using microbial lipids as biofuel is advantageous in microbes that have short life cycles, are easy to scale up, and require minimal labor. Mangrove forests, stretching along thousands of hectares of UAE coastlines, represent a unique ecosystem that harbors a diverse group of photosynthetic organisms with the potential to produce biofuel. In this study, we have identified fungal species obtained from the mangroves in Ras Al Khaimah capable of accumulating significant quantities of lipids that can be utilized as biofuel [2].
Living organisms synthesize organic compounds via the process of carbon dioxide fixation. It involves the addition of CO2 to ribulose 1,5-bisphosphate to produce two molecules of 3-phosphoglycerate, catalyzed by ribulose 1,5-bisphosphate carboxylase (rubisco). Rubisco is the most abundant protein on earth found in the stroma of the chloroplast. It initiates the Calvin cycle where CO2 is incorporated into organic molecules via light-induced reactions facilitated by ATP hydrolysis and NADPH reduction. Biomass accumulated due to CO2 fixation appears as algae mats, corn kernels, sugarcane stalks, and microorganisms’ lipid bodies [2]. Fungal oils are rich in polyunsaturated fatty acids (PUFAs). PUFAs are fatty acids with two or more double bonds between carbon atoms. They can be classified as ω (omega)-3, ω-6, ω-7, and ω-9 based on the location of their terminal double bonds. The biosynthesis of PUFAs involves polymerization of malonyl-CoA and acetyl-CoA to form fatty acids with as many as 22 carbon atoms such as docosahexaenoic acid. The pathway is catalyzed by several enzymes, including malic enzyme, acetyl-CoA carboxylase, adenosine monophosphate deaminase, and ATP-citrate lyase [2]. Oleaginous fungi possessing ATP-citrate lyase accumulate more lipids than those without the enzyme. Less than 50 fungal species can accumulate more than 25% of their biomass as lipids. Lipid accumulation is induced by surplus carbon and the lack of an essential nutrient (usually nitrogen) in the medium [3]. The absence of nitrogen prompts the accumulation of citrate, which is transported into the mitochondria in exchange for malate. The malate converts into pyruvate to generate NADPH used in fatty acid biosynthesis [4].
Although the first, second, and third generations of biofuels are available in the market, these biofuels have environmental and economic disadvantages. The first and second generations of biofuels involved the production of bio-ethanol. The first generation of biofuels threatens the food chain, lowers the soil quality, and reduces water availability for agriculture [5]. The second generation of biofuels presents a logistical, technological, and financial burden because of the required breakdown of resistant substances such as lignin [6]. The third generation of biofuels is derived from algae and represents a suitable source of biofuel due to their high lipid content and high productivity [2]. However, needed pretreatment in this biofuel category involves steps with high energy demands and the use of expensive chemicals that need to be curtailed to reduce the cost [7].
Microorganisms such as bacteria, fungi, and micro-algae that can produce and accumulate lipids are known as oleaginous microorganisms. Several studies have suggested that the next-generation biofuels could be made from the lipids stored by these microorganisms [8, 9]. Microbes store lipids as triglycerides (biofuel precursors) which are converted to fatty acid mono alkyl esters. Fungi are known to be capable of accumulating the most lipids among microbes. Lipid production can be maximized in oleaginous fungi by optimizing growth conditions and/or mutating enzymes involved in lipid synthesis [8, 9].
Fungi from mangrove ecosystems have not significantly been studied for SCOs production despite being the second largest group of marine fungi. In the present study, we have analyzed soil samples from the mangrove wetlands in Ras Al Khaimah, UAE, for the presence of oleaginous fungal species. We have further characterized the isolated fungi through visual identification and assessed their SCOs content by gravimetric analysis. In addition, lipid accumulation was examined under fluorescent microscopy.
2. MATERIALS AND METHODS
2.1. Sampling
Soil samples were collected with a spatula from different points nearby the plant roots (rhizosphere). In addition, random samples of soils were collected from the surface. The samples were obtained from an area (latitude: 25.77176; longitude: 55.943788) adjoining the Police Officers Club in the mangroves of Ras Al Khaimah.
2.2. Isolation of Fungi
Approximately 1 g of each soil sample was dissolved in 50 ml of Czapek Dox broth (bioWORLD) and incubated on a rotary shaker (180 rpm) for 72 hours at 28°C. Then 1 ml of the broth soil culture was plated on Czapek Dox agar (bioWORLD). Czapek Dox broth: (g/l), 30.0 sucrose, 3.0 NaNO3, 1.0 K2HPO4, 0.5 MgSO4.7H2O, 0.5 KCl,0.01 of ferrous sulfate supplemented with 15 g L-1 of NaCl, 6.7 mg /l of ZnSO4.7H2O and 1 mg/l, Co(NO3)2.6H2O [10].
2.3. Cultures to Screen Lipid Producing Fungal Isolates
The strains of interest cultured in Czapek Dox agar petri dishes were reinoculated in a lipid fermentation medium (Sigma-Aldrich®, Merck & Co, Scharlau and Himedia), mixed properly in a vortex mixer, and allowed to stand. After one hour, the broth cultures were plated on lipid fermentation agar plates (Sigma-Aldrich®, Merck & Co, Scharlau and Himedia) and kept at RT for five days. Morphologically distinct fungal colonies were identified and cultured in a one liter broth culture with a lipid fermentation medium. Lipid fermentation medium: (g/l), 30.0 glucose, 1.5 yeast extract, 15.0 NaCl, 0.5 NH4Cl, 5.0 Na2HPO4,12H2O, 7.0 KH2PO4, 1.5 MgSO4.7H2O, 0.1 CaCl2.2H2O, 0.01 ZnSO4.7H2O, 0.08 FeCl3.6H2O, 0.1 CuSO4.5H2O, and in mg/l, 0.1 Co(NO3)2.6H2O, 0.1 MnSO4.5H2O and pH adjusted to 5.5 [10].
2.4. Fluorescent Microscopic Examination of Lipid Accumulation
The fungal cultures were prefixed, harvested, and washed to visualize the lipid bodies (LBs) under the microscope. First, 1.34 ml of 4.8% formaldehyde (Merck & Co) containing 50 mM potassium phosphate buffer (Sigma-Aldrich®) [pH 6.8] and 0.5 mM MgCl2 (Scott science) (a.k.a. the formaldehyde stock solution) was used to prefix 10 ml of the culture. The culture was incubated on a rotary shaker (WiseShake®) (250 rpm) for 1 hour at 28°C. Second, the prefixed cells were harvested and resuspended to an OD600 (Labomed Inc) of 2.5 in the formaldehyde stock solution, followed by a 5-hour incubation at room temperature. Finally, the harvested cells were washed twice with 50 mM potassium phosphate buffer [pH 6.8]. To store the cells, we kept them in 100 mM potassium phosphate buffer [pH 7.5] at an OD600 of 2.5 at 4°C. Finally, each fungal culture was stained with the Nile red (TCI, Tokyo chemical industry) (9-diethylamino-5H-benzo[α]phenoxazine-5-one) dissolved in acetone (Merck & Co) at 0.1 mg/ml and observed under an Optika® fluorescence microscope to visualize the lipid bodies [10].
2.5. Extraction and Quantitative Estimations of Lipids
The harvested and washed cell mass was freeze-dried and disrupted using liquid nitrogen, and the dried biomass was finely crushed with 10 ml of methanol (Fisher chemical) in a chilled mortar and pestle. The dry weight (g) of crushed biomass was measured using the gravimetric analysis method to measure the biomass weight. The resulting homogenate obtained by crushing the biomass was processed in a glass stoppered flask as follows [11]:
20 ml of chloroform (Fisher chemical) was added to the 10 ml methanol homogenate to obtain a 2:1 (v/v) ratio of chloroform/methanol, and the suspension was stirred for 1 hour on a flat-bed stirrer at room temperature. The suspension was filtered through a sintered glass funnel and washed with 10 ml of 2:1 (v/v) chloroform/methanol. After filtration, 10 ml of 0.034% MgCl2 was added to the suspension in a 250 ml glass beaker, and the suspension was stirred for 10 minutes. In the next step, the suspension was centrifuged using a table-top centrifuge (Hettich) in glass vials at 3000 rpm for 5 min. The upper aqueous layer was formed as the centrifugation result was discarded, and the organic phase was washed with 10 ml of 4:1 (v/v) 2N KCl/methanol (KCl from Daejung chemicals). This step was repeated, but this time, the organic phase was washed with 10 ml of artificial upper phase (i.e., chloroform/methanol/water in a 3:48:47 ratio per volume). The suspension was subjected to the third round of centrifugation at 3000 rpm for 5 minutes. The aqueous and the protein layers at the phase boundary were discarded by aspiration. The organic phase was washed (as in the previous step) with an artificial upper phase until a clear phase boundary was obtained. The organic phase was transferred to a round-bottom flask of a rotary evaporator (Buchi), and the solvent was evaporated at 55°C. The weight of the extracted oil was measured, and the oil percentage in the dry biomass was calculated.
3. RESULTS AND DISCUSSION
The visual identification of the fungal cultures revealed that the species in our study were filamentous fungi, namely Aspergillus flavus (A. flavus) and Aspergillus niger (A. niger). A. flavus appeared as dark green colonies bound by a white ring (Fig. 1). A. niger had a starkly different appearance, with a dark brown lawn covering the entire surface of the medium (Fig. 2).
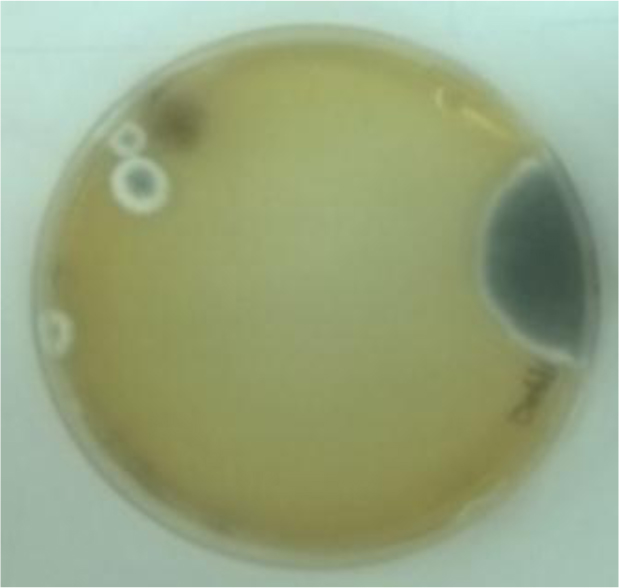
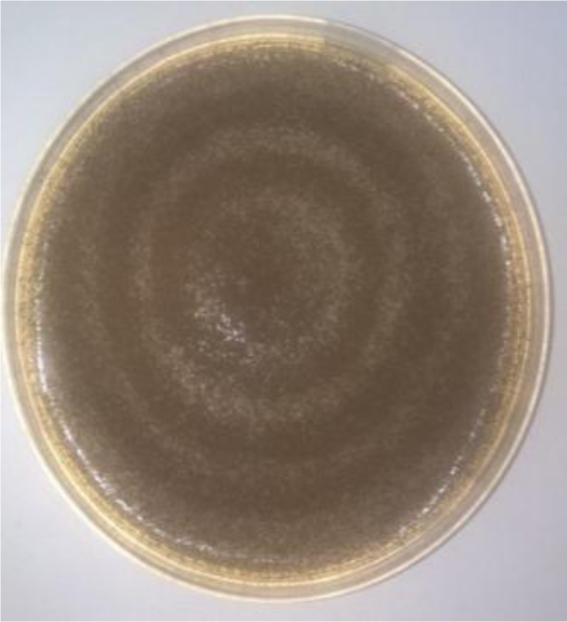
Extraction and gravimetric determination of lipid bodies indicated that A. flavus and A. niger yielded reasonable amounts of lipid bodies. A. flavus was estimated to accumulate 25.21% (w/w) while A. niger accumulated 24.34% (w/w) of their dried biomass as lipids.
A carbon-rich low-nitrogen lipid fermentation medium was used for fungal isolatesto trigger lipid accumulation. The lipid contents observed for A. flavus and A. niger were above 20%, therefore, within the average range of lipid content for oleaginous fungi. As shown in Fig. (3), in which Aspergillus flavus was stained with Nile red and visualized by fluorescence microscopy, lipid bodies (LBs) appeared as bright green spots. This observation demonstrated the promising potential of the fungal species under investigation for biofuel production. Other studies have revealed that some Aspergillus species can accumulate up to 51% (w/w) of their cell mass as lipids [10]. This level of lipid production may be achieved by optimizing the cultivation time, composition, and initial pH of the lipid fermentation media.

CONCLUSION
Fungal growth is very fast and is not impacted by seasonal, climate, and space variations as opposed to plants and animals. Fungi depends on cheap sources of nutrients such as waste, byproducts, and raw materials to synthesize single cell oils (SCOs). The percentages of oil content of the filamentous fungi A. flavus and A. niger indicate that these microorganisms are promising sources of next-generation biofuels. In future studies, we will focus on the fatty acid profiles of fungal SCOs. The fatty acid composition of SCOs will be analyzed by fractionation using silicic acid column chromatography for all the mangrove fungi oleaginous isolates to validate and compare their potential as biodiesel feedstock. Fungal SCOs will be assessed for their high fraction of saturated and monounsaturated fatty acids, primarily of the C16 and C18 series, which serve as a potential indicator of fungal-based biofuel quality.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This study was funded by Sheikh Saud bin Saqr Al Qasimi Foundation for Policy Research (Research Grant 2015-2016) and American University of Ras Al Khaimah (AURAK) (AURAK Research Seed Grant 2014).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We sincerely thank the Sheikh Saud bin Saqr Al Qasimi Foundation for Policy Research as they supported us by providing an important and highly competitive international grant. We would also like to thank the American University of Ras Al Khaimah (AURAK) for supporting our project with a seed grant.


