All published articles of this journal are available on ScienceDirect.
Plant-based Remedies with Reference to Respiratory Diseases – A Review
Abstract
In the era of air pollutants, respiratory diseases are a very common diagnosis in children, adolescents, and adults. Disorders of the respiratory system can affect both upper and lower respiratory system, and cause an immense worldwide health, economical and psychological burden.
Considerable attention is drawn to the use of plant-based products for the prevention and cure of health challenges, with respect of their eco-friendliness and very few side effects. Exposure to nature and active plant interaction is considered beneficial to physical and mental health. Plant-based drugs primarily target the immune and cardiovascular systems. Biologically active substances with different value can be identified from both terrestrial or marine botanicals, whose therapeutic abilities are an efficient control of an array of diseases.
In view of the potential of plant agents to positively influence respiratory diseases, this review will provide the reader with recent objective findings in the field of plant therapy and pharmaceutical agents and their ability to alter the physical and psychological complications of airborne diseases.
1. INTRODUCTION
Air pollution is defined as any harmful substance in the air that may cause problems to humans, animals, vegetation, or materials [1], and is estimated as the largest single environmental risk to health, responsible for numerous yearly premature deaths and disability globally [2]. Atmospheric contamination is directly linked to environmental degradation and has impacts on natural ecosystems and biodiversity by damaging crops, forests and other vegetation. People are constantly exposed to air pollution, and although it affects each individual, there are more vulnerable groups i.e., those with pre-existing health problems, children, elderly, and pregnant women. It enhances the severity of respiratory infections, such as asthma, and Chronic Obstructive Pulmonary Disease (COPD), in children and adults [3].
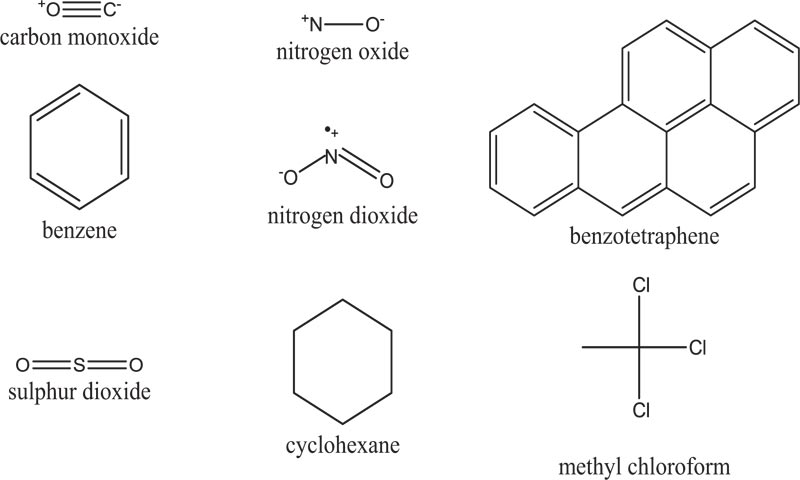
Air pollutants can be classified in various ways. Some authors have chosen four primary categories: chemistry, source, physical nature, and particle size [4]. Key primary air pollutants (Fig. 1) include particulate matter, black carbon, sulphur oxides, nitrogen oxide, ammonia, carbon monoxide, methane, non-methane volatile organic compounds, certain metals, and polycyctic aromatic hydrocarbons.
The human respiratory system is a biological system that consists of upper and lower parts. The upper respiratory system comprises of the nasal cavity, paranasal sinuses, and the pharynx, while the lower - the larynx, trachea, bronchi, bronchioles, and alveoli. Respiration itself refers to external and internal processes. External respiration acts for the exchange of O2 and CO2 between the body’s interstitial fluids and the external environment, while internal respiration represents the absorption of O2 and release of CO2 by cells. Disorders of the respiratory system can affect both upper and lower respiratory system (Table 1), and cause an immense worldwide health burden. Costs for the treatment of respiratory diseases by countries’ healthcare systems are an extensive weight on the economy.
| Disorder | Type | Affected Part of The Respiratory System | Symptoms | Triggers |
|---|---|---|---|---|
| Asthma | Chronic | Lungs | Intensity variable recurrent episodes of wheezing, breathlessness, chest tightness and/or coughing | Dust, smoke, pollen, volatile organic compounds |
| Bronchitis (acute) | Infectious | Trachea, bronchi | Persistent cough; occasionally sputum, dyspnea, wheeze | Pathogens, biomass fuels, dusts, chemical fumes |
| COPD | Chronic inflammatory | Lungs | Persistent cough with mucus production, dyspnea, wheezing, chest tightness | Smoking, chemical fumes, dusts, other lung irritants, α-1 antitrypsin deficiency (rarely) |
| Common cold | Viral infection | Nose, sinuses, pharynx, larynx | Sore throat, rhinitis, rhinorrhea, cough, malaise | Rhinovirus, bacteria |
| Cystic fibrosis | Chronic genetic progressive |
Lungs | Coughing, wheezing, repeated lung infections, shortness of breath, labored breathing | - |
| Emphysema | Chronic progressive | Lungs | Shortness of breath | Active proteolytic enzyme |
| Hay fever (Allergic rhinitis) | Inflammatory | Nose | Sneezing, sore and runny eyes, nasal itching congesting, sinus pain, tickly or itchy throat | Pollen, mites, domestic animals, molds, tobacco smoke, automobile exhaust |
| Influenza | Viral infection | Lungs, nose, throat | Weakness, myalgia, cough, nasal congestion | Viruses |
| Lung cancer | - | Lungs | Worsening cough, hoarseness, tiredness, chest pain | Smoking, genetic predisposition, repetitive respiratory infections |
| Pulmonary fibrosis | Idiopathic | Lungs | Cough, shortness of breath, fatigue, low saturation levels | - |
| Pneumonia | Infectious, inflammatory |
Lungs | Fever, cough, dyspnea, chest pain | Virus, bacteria, fungi, parasites |
| Respiratory distress syndrome | Inflammatory | Lungs | Dyspnea, tachypnea, hypoxemia | Viruses, fungi, parasites, bacteria |
| Sinusitis | Chronic inflammatory | Sinuses | Facial pain/pressure, facial congestion/fullness, nasal obstruction | Allergens, viruses, fungi, bacteria, animal dander, polluted air, smoke, dust |
Asthma is one of the world’s most common chronic diseases. It is defined as an inflammatory disease of the lower airways. Asthma is associated with T helper cell type-2 immune responses, and is not currently curable [17]. The diagnosis can be suspected if the patient has a medical history of recurrent dry coughing, rhonchus, wheezing, chest tightness, or shortness of breath [18]. Allergic rhinitis (hay fever) is a disorder of the nose provoked by allergen exposure triggering IgE-mediated inflammation [19]. It is mostly known by four major symptoms (rhinorrhea, sneezing, nasal itching, and nasal congestion) and the condition worsens in the presence of asthma. Sinusitis is a very common chronic illness diagnosed by general practitioners exerting in the inflammation of one or more of the paranasal sinuses. The occurrence of rhinosinusitis is higher in patients with allergy, particularly those with IgE mediated allergic rhinitis [20, 21].
Bronchitis is a respiratory infection characterized by increased mucus production and inflammation in airways [22]. It is difficult to be distinguished from other illnesses that commonly cause cough i.e., influenza, common cold, etc [23]. The common cold is a conventional term for a mild self-limiting viral infection of the upper respiratory tract [24]. Influenza is a viral infection of the respiratory tract that occurs in distinct out-breaks each year [25]. Acute respiratory distress syndrome is a life-threatening inflammatory lung injury [26], which occurs with acute respiratory failure as a result of clearly determined pulmonary and non-pulmonary insults [27]. Emphysema is the chronic shortness of breath and inability to tolerate physical activity because the respiratory membrane has been damaged. Evidence shows an increased number of neutrophils, macrophages, T-lymphocytes and eosinophils in the emphysematous tissue in the pulmonary parenchyma and in the terminal air spaces in the lungs [28]. COPD is a poorly reversible lung disease characterized by narrowing of the airways [29]. In larger airways, the inflammatory response is referred to as chronic bronchitis, whereas in the tiny air cells it leads to emphysema [30].
Cystic fibrosis is a progressive, chronic genetic disease which is characterized by thick mucus development and lost lung tissue elasticity. It is caused by a mutation in the gene that disrupts the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR is responsible for the regulation flow of salt into and out of cells, which is required for the generation of normal mucus. Consequently, cystic fibrosis causes lung complications i.e., bacterial infection, inflammation, and lethal development [31]. Pneumonia is a serious infection of the lower respiratory tract (lungs) with high mortality and morbidity. It can be acquired in the community, the hospital environment, and can be transmitted by the aspiration of a pathogenic microorganism or by inhalation of a pathogenic microorganism. Streptococcus pneumonia (pneumococcus) is the most common pathogen causing community-acquired pneumonia [32]. Pulmonary fibrosis is a lung disease characterized by identifiable or more commonly unknown irritation to the lungs [33]. Existing studies have pinpointed that exposure to wood and metal dust, pollution, gastric aspiration, smoking, and infection are factors that may contribute to an increased risk of developing pulmonary fibrosis [34].
Disease-control phytotherapy is well-known since the ancient civilizations [35]. The term phyto-pharmaceutical derives from phytón (Greek for plant) and phármakon (Greek for medicine). Herbal remedies are prepared to treat different conditions or illnesses. Different solvents can be used, as well as drug-extract ratios and plant parts i.e., leaves, bark, or roots [36]. A large portion of the developed countries’ populations cherishes herbaceous plants for basic health care [37]. Furthermore, the WHO reports that 80% of the world’s population satisfies their primary health related needs with the use of phytochemistry and 11% of drugs originate from plants [38]. Natural biological compounds are associated with limited side effects and reduced costs in the production of anti-inflammatory drugs [39]. The pharmacological benefits of medicinally important plants are basically due to the structurally diverse bioactive phytochemicals, i.e., flavonoids, phenolic acids, anthocyanins, etc., produced in the plant as primary/secondary metabolites [40]. Reasonable attention is presently given to the use of eco- and bio-friendly plant products in the prevention and cure of health challenges [41]. Phytopharmaceuticals contain pharmacologically active ingredients that interact with the human body’s protein structures [42]. This differentiates them from the homoeopathic remedies that are highly diluted, and the actual active ingredient is in very small quantity.
The growth of the pharmaceutical herbal extracts market is mainly due to the availability of the herbs, which makes them easy to reach and extract, as well as cost efficient. A major challenge for the extraction, though is the standardization and quality control. For instance, harvesting time, climate change, storage process, etc. can all affect the quality of the final plant preparation. The extraction is either single herb based or multiple herbs based. Thus, standardization is difficult with the pharmaceutical herbal extraction compared to the chemical compositions.
In view of the potential of plant agents to positively influence respiratory diseases, this review will provide the reader with recent objective findings in the field of plant therapy and pharmaceutical agents and their ability to alter the physical and psychological complications of airborne diseases.
2. BRIEF HISTORY OF AIRBORNE EPIDEMIC EVENTS
Throughout history, infectious diseases have been the deadliest weapon for human beings. There are same notorious epidemics that have changed the perception for health and safety (Table 2). The Oxford Dictionary defines an epidemic as “a temporary prevalence of a disease”. Endemic is probably most frequently used to describe a disease that is prevalent in or restricted to a particular location/region/population. In the 1600s, the Latin term “pandemic” has also entered the English terminology. Pandemic derives from the Greek “pándēmos”, meaning common/public. As an adjective, pandemic can also mean general/universal, also often with a negative connotation. However, pandemic is usually contexted with an epidemiology, concerned with infectious diseases.
| Year | 1918 | 1957 | 1968 | 1977 | 2002 | 2009 | 2019 |
|---|---|---|---|---|---|---|---|
| Event | Spanish flu | H2N2 | H3N3 | H1N1 | SARS | H1N1 | SARS-Cov-2 |
The 1918 influenza pandemic is the most severe pandemic in recent history with an estimated of approximately fifty million deaths worldwide [43]. The impact of this pandemic, known as the Spanish flu, was not limited to 1918-1919. Since then, all influenza-A pandemics, have been caused by descendants of the 1918 virus. In 1957, a new influenza H2N2 virus emerged in East Asia, triggering the Asian flu pandemic. It was initially reported in Singapore in February, and in Hong Kong in April. Later, in summer, it had spread in the United States and the UK resulting in a total of 100 000 death cases [44]. This H2N2 virus was comprised of three different genes from an H2N2 virus, including the hemagglutinin (H2) and the neuraminidase (N2) genes. The 1968 pandemic, first noted in China in July, and the United States in September, was caused by the H3N2 virus comprised of two genes from an avian influenza A virus, including a new H3 hemagglutinin (H3), and the neuraminidase (N2) from the 1957 H2N2 virus. Statistics show it has killed between one and four million people. The H3N2 virus is still in circulation today and is considered to be a strain of seasonal flu [45]. The Russian Flu (red influenza or red flu) first came to attention in 1977 when H1N1 viruses were isolated in northern China, and spread rapidly in the Soviet Union causing an epidemic disease in children and young adults worldwide. In the beginning of 1978, the virus had spread round the world [46]. In 2002, the first coronavirus outbreak was registered, when the Severe Acute Respiratory Syndrome (SARS) emerged in China and spread worldwide, causing more than 700 deaths. In 2009, a novel influenza A (H1N1) virus emerged, first detected in the United States, and then quickly spread to the world. The virus ((H1N1)pdm09) was known as a combination of influenza genes not previously identified in animals of humans. In 2019, another coronavirus outbreak was registered (SARS-Cov-2), which was the third coronavirus eruption in twenty years. The virus is 96.2% identical to a bat CoV RaTG13, thus it can be assumed that the virus originally came from bats and has been transmitted over time to other animal hosts and ultimately to humans [47]. The ongoing pandemic has reached nearly 40 million cases and 1 320 000 deaths [48].
Serious consequences, through multiple channels, from pandemics exist. The economic consequences of the pandemic include labor shortages and wage increases, as well as an increased use of social security systems. Pandemics can cause economic damage, including short-term fiscal shocks and longer-term negative shocks to economic growth [49]. Regions that are more economically integrated to the world via international trade are more affected by the pandemic situations [50]. People are at risk of both physical and psychological complications [51]. General psychiatric symptoms during outbreaks range from 17 to 75%. People tend to feel anxious and unsafe when the environment changes and their response to fear of uncertainty leads to negative societal behaviors [52]. Fear and isolation of those who are sick/quarantined, disruption of everyday life, and mental health impacts are real outcomes of a pandemic [53]. Individual behavioral changes, i.e., fear-induced aversion to workplaces and other public gathering places, are a primary cause of negative shocks to economic growth during pandemics. Up to 70% of health care workers (physicians, nurses, and auxiliary staff) suffer from traumatic stress symptoms, i.e., depression (more than 30%), insomnia (approximately 35%), anxiety (45%) lasting up to three years [54].
3. IMMUNE SYSTEM AND SUPPORT
The immune system is composed of a complex network of innate and adaptive components that are able to protect the host from a universe of pathogenic microbes, and are themselves constantly evolving. The immune system also eliminates toxic or allergenic substances that enter the host through mucosal surfaces [55]. The alveolar surface is in direct contact with the external environment, and is constantly exposed to invading microorganisms [56]. There, a layer of pulmonary epithelial cells plays a physical and biological barrier for inhaled substances and pathogens. The mucus coating of the pulmonary epithelium, proteolytic enzymes, defense proteins and lysozymes present on the surface and in the fluids around alveoli protect against infection [57]. Pulmonary epithelial cells are in close contact with cells of the immune system, and express cell surface receptors, which enable them to recognize pathogen-associated molecular patterns from viruses, bacteria, fungi, protozoa, and multicellular parasites [58].
The adaptiveness of the immunity is associated with the acquisition of a complex microbiota. The microbiota plays a fundamental role on the induction, training and function of the host immune system [59]. The innate immunity of the lung is represented by infiltrating (e.g., neutrophils) and resident (e.g., macrophages or innate lymphoid cells) immune cells [60]. The lower respiratory tract microbiome forms within the first two postnatal months, alongside with lung immune maturation [61]. The use of antibiotics and changes in diet, drastically change the components of the human microbiota [62]. It was thought that the lung was largely sterile, but a recent description of the resident respiratory microbiota shed light to the etiology of asthma and other airway inflammatory disorders, such as cystic fibrosis [63]. Rising evidence, emphasize the gut-lung axis’ – an important crossroad between gut and lung microbiome. At the phylum level, Firmicutes, Bacteroidetes and Proteobacteria are the most common phyla [64]. Lung immunity and its response to Influenza A infection is related to the ability of the gut to regulate the antiviral immunity [65] through the production of desaminotyrosine, initially deriving from the gut commensal Clostridium orbiscindens [66]. The exposure to Pseudomonas and Lactobacillus enhances a Th17 type response [67] and Proteobacteria induce severe TLR2-independent airway inflammation [68]. In the event of lung pathology, severe changes in the local environment occur, creating a favorable environment for a specific microbial growth. When asthma is diagnosed, the bronchoscopy shows the exclusive presence of Haemophilus, Neisseria, Fusobacterium, and Porphyromonas [69, 70]. In chronic obstructive pulmonary disease, Lactobacillus, Fusobacteria, Leptotrichia and Fusobacterium were observed; and Pseudomonas, Staphylococcus, Stenotrophomonas, Achromobacter, and Streptococcus were the most present in cystic fibrosis [71-74]. Respiratory viral infections i.e., rhinovirus, influenza virus, adenovirus, and parainfluenza virus are quite common during childhood and often increase susceptibility to secondary bacterial infection of the lungs [75].
Thymus vulgaris L. (A), Malva sylvestris L. (B), Sambucus nigra L. (C)
4. PLANTS AS BENEFICIAL AGENTS
Exposure to nature and active plant interaction is considered beneficial to physical and mental health through the promotion of comfortable, soothed, and natural feelings, as well as suppression of the sympathetic nervous system activity and diastolic blood pressure [76, 77]. Plants are the most abundant natural element and are frequently regarded as the most representative of natural landscape [78]. However, indoor plants are different from the natural outdoor environment. Thus, indoor plants can be perceived as a symbol of nature or as a result of human interaction with nature [79]. Even limited number of indoor plants can lead to the reduction of sick leave days and promote a feeling of preference, comfort, and wellbeing [80]. Authors report a positive correlation between the visible density of urban tree coverage with stress recovery [81] and landscape preference [82]. Indoor plants that can regulate indoor air quality and microclimates without consuming energy warrant greater attention and wider application. Plant species and cultivars help with volatile organic compounds removal [83]. The differences between plants lead to different volatile organic compounds removal abilities - leaf parameters i.e., stomatal characteristics, wax layer, and hair growth all influence the volatile organic compounds diffusion into the leaf [84]. An investigation of Yang et al. [85] points out that members of the Araliaceae family had a tendency towards intermediate to high volatile organic compounds removal rates, whereas members of the Araceae family exhibited lower removal rates. Kim et al. [84] studied formaldehyde removal rates and reached to the conclusion that ferns had the highest formaldehyde removal rates followed by herbs. The potentially beneficial influences of vegetation on air quality are categorized under the processes of dry deposition and atmospheric dispersion [86]. Dry deposition looks at the process where pollutants are at least temporarily removed from the ambient air with the help of interception, sedimentation, capture, biochemical processes, etc [87]. Green infrastructure can reduce pollution exposure at the local scales. Numerous studies have discovered that people who live in particularly quiet places (rural areas, large green areas) have a better quality of life [88]. Urban vegetation can absorb the noise of various human activities by making a certain contribution to acoustic health [89]. Green spaces that can offer conditions of thermal well-being by allowing a higher water penetration into the ground, and making it available for evaporation and therefore contributing to further reduce the ambient temperature [90].
5. PLANTS AND THE PHARMACEUTICAL INDUSTRY
Phytochemicals occur naturally in the plant kingdom and have proven their therapeutic value as a source of a wide spectrum of biological activity [91, 92]. Plants contain various active ingredients (Fig. 2) based on which their usefulness and potential therapeutic effect can be speculated. The structures of secondary metabolites act as defense mechanisms within the cell in herbivores, microbes, and plants by interfering with molecular targets [93].
The identification of as many chemical entities and groups, for instance, phenol and organic acids, carbohydrates, amino acids, etc is very valuable for the development of plant-based drugs and pharmaceuticals [94]. Biologically active substances can be identified from either terrestrial or marine botanicals and their value depends on the properties of the identified substances [95]. Both nutritional and therapeutic abilities of plants result in and efficient control of an array of diseases [96]. Plants are also essential for the ecosystems by showing their flexible ecomorphology. Plants are directly linked to the development of animals, humans, insects, and general ecosystem health. Herbal agriculture and farming are becoming an area of extensive research [97]. Environmental factors (climate, soil, geography) influence directly or indirectly plant development [98]. Unfortunately, as a result of over-harvesting, loss of habitat, lack of sustainable agriculture, and conservation practices, many medicinal plants are facing extinction. However, plants can overcome numerous constrains (physical, biological, chemical) by regulating their built-up secondary metabolites [99, 100]. Today, the pharmaceutical industry is one of the economically thriving industries throughout the world. Of the estimated between 215,000 and 500,000 higher plant species worldwide, a limited 6% have been screened for their biological activity [101]. In Europe, more than 2,000 medicinal and aromatic plants are commercially traded, and around 10% of those plant species are threatened in at least one European country by this trade [102]. More than 82% of drug substances are directly isolated from natural products and nearly 50% are coming from plant/herb isolated compounds [103, 104]. Plant-based drugs primarily target the immune system, cardiovascular, antimicrobial and anticancer support. Pharmaceutical crops are gaining popularity, although conservation biologists and ecologists have paid attention on the biosafety and environmental impacts of genetically modified crops [105] since pharmaceutical crop biotechnology and biodiversity conservation are not mutually exclusive. Biotechnology is a powerful tool to obtain plants bioactive compounds [106]. Different strategies can be applied to improve the production of compounds of interest, some of which may include plant cell culture, gene expression modification, etc [107]. Some bioactive compounds can be greatly enhanced. Grasses usually contain the types of secondary metabolites that are regarded as biologically inactive. However, Cymbopogon spp. is found to be rich in phytochemicals - total soluble phenolics, flavonoids, iridoids and proanthocyanidins [108]. Alkaloids, abundant in plants, have demonstrated significant anti-inflammatory [109] and antibacterial [110] activity, as well as stimulatory effect on the β2-adrenoreceptors in the trachea and may contribute to being a potential therapeutically effective antitussive against several forms and etiologies of cough [111]. Several plants from the Amaranthaceae and Zingiberaceae families have been reported as effective in the treatment of respiratory diseases (e.g. asthma, cough) [112]. Plant parts (leaves, seeds, flowers, roots) are prepared differently (decoction, infusion, and powder) to administer the active ingredients for the treatment of respiratory diseases [113]. Many companies put effort in the development of drugs with natural active ingredients. Table 3 visually proves information about some of the popular medical preparations targeting problems with the respiratory system [114-149]. Common ingredients in plant-based drugs are Thymus vulgaris L, Sambucus nigra, Allium spp., Belladonna, Pulsatilla, Arnica montana L., etc.
The plant extracts market is estimated at USD 23.7 billion in 2019 and is projected to reach USD 59.4 billion by 2025 [150]. Comprehensive data supports the use of preparations to relief some of the most common symptoms of respiratory diseases i.e., sore throat, cough, nasal congestion, etc [124]. Herbal sources have been reported individually or in combinations in the available literature. The use of homeopathy is much disseminated and positive experience is associated with its use. What is more, the immerging resistance to antibiotics, an alternative is much needed, which consequently rises the use of homeopathic drugs. Nevertheless, several limitations (exact mechanism of action and dosage) exist when it comes to using preparations with natural ingredients [151]. A major trend in pharmaceutical herbal extracts market is the combining herbal medicine with nanotechnology.
| Drug Name | Type | Used to Treat | Natural Active Substances | Active Substance | IC50 | Marketing Authorization Holder |
|---|---|---|---|---|---|---|
| Prospan | Syrup | Relieve coughs, loosen mucus & phlegm and alleviate the symptoms of chronic bronchitis | Hedera helix L., folium leaf extract | Hederacolc-hiside α-hederin β-hederin |
1.2 μM 13.6 μM 12.0 μM |
Engelhard Arzneimittel GmbH & Co. KG |
| Koflet | Syrup | Reduce the viscosity of bronchial secretions and facilitate expectoration | Mel despumatum; Syzygium aromaticum; Cinnamomum zeylanicum; Ocimum basilicum; Glycyrrhiza glabra; Zingiber officinale; Piper longum; Vitis vinifera; Malva sylvestris | Data not available | The Himalaya Drug Company | |
| Septilin | Tablets | Respiratory tract infections | Conch shell and Indian Bdellium; Maharasnadi Kwath, Manjistha, Tinospora, Amla, Yashtimadhu, Shigru | Data not available | ||
| N-Ti-Tuss | syrup | Different types of coughs |
Terminliâ belerika; Licorice, Vasika Adatoda; Sacred Basil; Turmeric; Galangala; Long pepper; Eucalyptus oil |
Curcumin | 50 -100 μM | Ecopharm |
| Hustagil | Syrup | Bronchi inflammation and symptoms of bronchitis | Thyme | Carvacrol thymol |
7.2μg/mL 9.8μg/mL |
Dentinox Gesellschaft für pharmazeutische Praparate |
| Mucoplant | Syrup | Respiratory diseases; Chronic airway obstruction in children |
Ivy leaf dry extract | Hederacolc-hiside α-hederin β-hederin |
1.2 μM 13.6 μM 12.0 μM |
Dr. Theiss Naturwaren GmbH |
| Aflu Drops | Drops | Flu-like symptoms |
Aconitum napellus; Baptisia tinctoria; Eupatorium perfoliatum; Gelsemium sempervirens |
Data not available | ||
| GrinTuss | Syrup | Dry and chesty cough | Grindelia, plantain | Quercetin-3-methylether | 19 μM | Aboca S.p.A. Società Agricola |
| Sinupret | Tablets | Acute and chronic inflammations of the paranasal sinuses | Primula veris/elatior; Gentiana lutea; Sambucus nigra; Rumex species; Verbena officinalis | Nigrin f verbascoside verbenalin hastatoside |
1.8 -3.7 ng/ml N.A. |
Bionorica SE |
| Tonsipret | Tablets | Severe sore throat | Paprika, pock wood, pokeweed | Data not available | ||
| Sinulan | Tablets | Nasal congestion and sinus problems | Gentiana lutea; Verbascum thapsus; Sambucus nigra; Verbena officinalis; Andrographis paniculata | Isogentisin nigrin f |
39.6 μg/mL 1.8 -3.7 ng/ml |
Walmark |
| GeloMyrtol | Tablets | Treatment of bronchitis and sinusitis (acute and chronic) | Eucalyptus, sweet orange, myrtle and lemon | Myricetin eucalyptol α-pinene |
40µg/mL N.A. |
Nadurel Pharma |
| Sinnabsin | Tablets | Acute and chronic rhinosinusitis | Cinnabaris; Hydrastis; Echinacea |
Data not available | Deutsche Homoopathie-Union DHU-Arzneimittel GmbH & Co | |
| Influcid | Tablets | Feverish flu-like infections and other acute viral upper respiratory tract infections and for supportive treatment of influenza | Aconitum; Gelsemium; Ipecacuanha; Bryonia; Eupatorium perfoliatum | Lycoctonine anopterine napelline polyphenols |
N.A. 7µg/mL |
|
| Rhinital | Tablets | Hay fever (seasonal allergic rhinitis/pollinosis) and non-seasonal allergic coryza (perennial allergic rhinitis) | Galphimia glauca; Cardiospermum halicacabum; Luffa operculata | Chrysoerisol, apigenin, luteolin |
29 μg/ml | |
| Tavipec | Tablets | Acute bronchitis, sinusitis and coughs | Lavender oil | Eucalyptol, linalool, α-terpineol |
N.A. | Pharm. Fabrik Montavit Ges.m.b.H. |
| Tussavit | Syrup | Expectorant in cough associated with cold | Thymus vulgaris L.; Thymus zygis L.; Plantago lanceolata L. | Carvacrol thymol |
7.2μg/mL 9.8μg/mL |
|
| Sinubell | Tablets | Allergic sinusitis | Sticta pulmonaria | Data not available | Bell Homeopathy Ltd. | |
| Asthnobell | Tablets | Respiratory failure caused by bronchitis or asthma |
Sambucus nigra; Badiaga; Antimonium tartaricum; Drosera rotifundifolia; Poumon histamine |
Nigrin f | 1.8 -3.7 ng/ml | |
| Engystol | Tablets | Common cold, flu | Vincetoxicum hirundinaria | Data not available | Biologische Heilmittel Heel GmbH | |
| Tartephedreel heel | Drops | Inflammation of the airways | Tartarus stibiatus; Hepatica triloba; Belladonna; Natrium sulfuricum; Arsenum jodatum; Quebracho; Betonica; Anisum stellatum; Lobelia inflate; Ipecacuanha; Blatta orientalis; Medorrhinum; Ephedra vulgaris | Lobeline Alkaloids |
N.A. | |
| Coryzalia | Tablets | Common cold and its symptoms (nasal congestion, sneezing, runny nose); rhinitis (acute, recurrent, infectious or allergic) | Allium cepa; Belladonna; Gelsemium sempervirens; Pulsatilla; Sabadilla | Quercetin, ishorhamnetin, kaempferol rutin, saponin D |
N.A. 9.37 µg/mL |
Boiron |
| Stodal | Syrup | Cold symptoms i.e., nasal congestion, runny nose, sneezing; minor sore throat, dry or productive cough, or chest congestion | Dulcamara; Hydrastis canadensis; Nux vomica | Hydrastine, berberine, sideroxylin, brucine, strychnine |
N.A. | |
| Paragrippe | Tablets | Symptomatic treatment of flu conditions (chills, muscle aches, fever, headache) | Arnica montana, Belladonna; Eupatorium perfoliatum; Gelsemium sempervirens | Helenalin rutin |
0.44 μM N.A. |
|
| Oscillococcinum | Pillules | Flu-like symptoms | Anas barbariae Hepatis; Cordis extractum | Data not available | ||
| RhinAllergy | Tablets | Symptoms (sneezing, runny nose, itchy and watery eyes and scratchy throat) caused by seasonal allergies (hay fever, pollen); environmental allergens (dust, mould, animal hair) | Allium cepa; Ambrosia artemisiaefolia; Euphrasia officinalis; Sabadilla; Solidago virgaurea | Quercetin, ishorhamnetin, kaempferol |
N.A. |
|
| Homeogene | Tablets | Sore throats, laryngitis, dysphonia | Mercurius solubilis; Pulsatilla; Spongia tosta, Bryonia; Belladonna; Phytolacca decandra; Arum triphyllum;Arnica montana | Helenalin |
0.44 μM |
Boiron |
| Roxalia | Tablets | Sore throat and hoarseness relief | Arnica montana; Arum triphyllum; Belladonna; Bryonia; Mercurius solubilis; Hahnemanni; Phytolacca decandra; Pulsatilla; Spongia tosta | Helenalin cucurbitacins |
0.44 μM N.A. |
|
| Sinusalia | Tablets | Relief of congestion and pain due to sinus inflammation | Belladonna; Sanguniaria canadensis; Spigelia anthelmia | Scopolamine, atropine, sanguirubine, chelilutine, allocryptopine |
N.A. 1.5μM |
|
| Pranarom aromaforce | Tablets | Sore throat | Lima; Cajeput, Peppermint; Tea Tree; Savory, Laurel; Ceylon cinnamon | Linalool, phenylethyl alcohol, citronellol | N.A. | Pranarom International |
| Gripp Heel | tablets | Cold and flu symptoms | Aconitum napellus; Bryonia cretica; Lachesis mutus; Eupatorium perfoliatum | Aconitine aconine |
N.A. | Heel Canada Inc. |
CONCLUSION
Plants and herbs attract great interest because of their unique abilities to enhance beneficial effects for the consumer with only a small quantity needed. The production of plant-derived pharmaceuticals is constantly evolving currently focusing on plant molecular farming and nanomedicine using traditional agents. With the constant advances in the fields of biotechnology and analytical chemistry, new bioactive substances are being identified in plants through both phytochemical and pharmacological studies. The concept of using plants in traditional medicine is not new. This leads to the significant constraint of inadequate quantities, needed for development and clinical use of new drugs with natural active ingredients, as well as the need for improved isolation extraction techniques. Nanotechnology provides great opportunities for the application and efficacy of plant-based remedies because of their low bioavailability. Homeopathy itself is an economic therapy choice because of the very small quantities of medicine being prescribed. Nanomedicine, combined with homeopathy, can provide important alternative health care agents from natural ingredients in favor of both developed and developing nations.
Numerous illnesses can be alternatively treated by immunomodulation using medicinal plants, instead of chemotherapy [152]. The discovery and isolation of more specific immunomodulatory agents from plant origin possesses potential to counteract the side effects and high cost of synthetic compounds.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Dr. Dasha Mihaylova is part of the Editorial Board of the Open Biotechnology Journal.
ACKNOWLEDGEMENTS
Declared none.


