All published articles of this journal are available on ScienceDirect.
Monoclonal Antibodies for Immunohistochemical Diagnosis of Breast Cancer
Abstract
Breast cancer is a leading malignant disease in women worldwide, although its pathology is visually localised. Currently, it has been proven that the parameters of molecular genetic biomarkers, including oncoprotein HER2, proliferation markers Ki-67, oestrogen receptors ER, and progesterone receptors PgR, are associated with breast carcinogenesis and are a reflection of the biological aggression of the tumour. The significance of these biomarkers in signalling pathways and genetic mechanisms of carcinogenesis has been described, as well as the relationship between the expression levels of each biomarker and the tumour response to appropriate therapy.
The primary antibody that imparts specificity to IHC is based on the monoclonal antibodies (mAbs) as the main immunoreagent that enables reliable identification of breast cancer cells. The most commonly used antibodies to molecular biomarkers for IHC were determined in accordance with indicators of laboratory use and efficiency (pass rate) of HER2, Ki-67, ER, PgR assessments in the NordiQC breast cancer module. The discovery of the complete structure of these biomarkers and the design of their domains and subdomains by genetic engineering methods enable the synthesis of effective monoclonal antibodies. Quantitative indicators of the expression levels of tumour biomarkers of breast cancer were determined using mAb, depending on epitope specificity and affinity.
1. INTRODUCTION
Breast cancer is the most diagnosed cancer among women in many countries. In 2020, new cases of breast cancer were detected, accounting for 24.5% of all 9 227 484 registered cases of cancer in women worldwide. Therefore, breast cancer is a leading malignant disease, comprising 11.7% of the total number of new 19 292 789 cases in 2020 [1].
The effectiveness of breast cancer treatment depends on the early diagnosis and stage of the disease. ImmunoHistochemical Analysis (IHC) is one of the most informative methods to confirm the risk of malignancy in humans. To individualize the treatment of cancer patients, pathomorphological laboratories carry out IHC testing to verify the diagnosis and the molecular characteristics of tumours. The use of molecular markers for IHC diagnosis of breast cancer complements the accuracy and reliability of morphological assessment of the degree of malignancy. The detection of cancer biomarkers involved in carcinogenesis provides additional information about tumour growth and its ability to invade and metastasize [2-4].
Standard morphological examination of primary tumour tissue of breast cancer includes IHC detection of oncoprotein HER2, proliferation marker Ki-67, oestrogen receptors (ER), and progesterone receptors (PgR) expression levels to assess prognosis and prescribe personalised therapy. This is due to the involvement of these biomarkers in signalling pathways and genetic mechanisms of carcinogenesis, as well as the relationship between each biomarker and the tumour in response to appropriate therapy.
2. STRUCTURE, FUNCTION, AND EXPRESSION OF MOLECULAR BIOLOGICAL MARKERS FOR IMMUNOHISTOCHEMICAL DIAGNOSIS OF BREAST CANCER
HER2 (human epidermal growth factor receptor 2 - ErbB2) is a 185-kDa cell membrane-bound transmembrane tyrosine kinase receptor that is a member of the epidermal growth factor receptor family composed of HER1, HER2, HER3, and HER4. This oncoprotein consists of three domains: An Extracellular Domain (ECD), a hydrophobic Transmembrane Domain (TMD), and an Intracellular Domain (ICD) tyrosine kinase (Fig. 1).
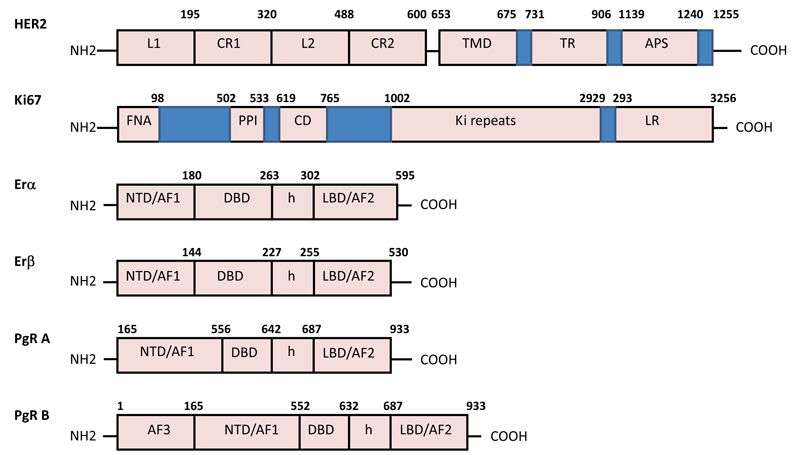
HER2 plays an important role in regulating cell proliferation, differentiation, and survival in normal cells. The ECD of HER2 takes part in signal transduction through dimerization with other receptors. It is involved in controlling the receptor activation, while the ICD is associated with cell growth and differentiation. Uncontrolled activation of HER2 receptors promotes the proliferation of tumour cell pathways, leading to their unrestricted growth and subsequent overexpression of HER2 [5].
The oncomarker HER2 is considered a factor of poor prognosis, and its high expression is an indicator of the highly metastatic ability of the tumour. Likewise, overexpression of HER2 is associated with a worse prognosis of the disease. Expression of HER2 is not observed in normal and dysplastic mammary gland tissue but is presented at the early stages of ductal carcinoma and is negatively correlated with hormonal status, high proliferation level, and aneuploidy. Additionally, its overexpression indicates the resistance of the tumour to chemotherapy. In addition to its diagnostic significance, HER2 protein overexpression is also a target for anticancer therapy, as targeted by the anti-HER2 monoclonal antibody Herceptin (Trastuzumab), used in breast cancer [6-8].
Ki-67 (biomarker cell proliferation) is a large nuclear protein existing in two isoforms, 345 and 395 kDa. The Forkhead-Associated (FHA) domain and the binding site for Protein Phosphatase I (PP1) are located on the N-terminus of the Ki-67 protein. This is followed by a conservative domain (CD) of 31 amino acids with unknown functions, and the largest Ki-67 domain is composed of multiple repetitive elements consisting of 122 amino acids in length with 43–62% homology. Within these “Ki-67 repeats”, there is a highly conservative 22–amino acid region known as the “Ki-67 Motif” with 90–100% identity. On the other hand, the C-terminus of the Ki-67 is enriched with pairs of leucine and arginine residues (LR domain) through which Ki-67 can bind to DNA (Fig. 1).
Ki-67 is expressed in the cell nucleus during the G1, S, G2, and M phases of the cell cycle, but not in G0. Upon transition to the G0 phase after mitosis, the Ki-67 antigen rapidly undergoes catabolism. Currently, there is no consensus on the role of Ki-67 protein during the cell cycle. Two main hypotheses were put forward regarding the role of Ki-67, stating that it is a protein regulator of the cell cycle and is a DNA-associated protein necessary for the organization of DNA structure [9].
Determination of the expression level of Ki-67 plays an important role in differentiating the molecular subtypes of breast cancer and is one of the criteria for determining the scheme of chemotherapy. Increased expression of Ki-67 is associated with a poor prognosis of breast cancer treatment and is closely related to growth and invasion, as the expression level of Ki-67 is very low in normal breast tissue. Ki-67–positive cases are more active in relation to growth, more aggressive in terms of invasion, and are characterized by more pronounced metastasis. Additionally, a high expression level of Ki-67 is associated with the recurrence rate and low overall survival of patients [10-12].
The nuclear oestrogen receptors (ER) and progesterone receptors (PgR) are composed of the identical C-terminal Ligand-Binding Domain (LSD), the central DNA-Binding Domain (DBD), and the aminoterminal domain (NTD). For interaction with cofactor protein complexes, there are two domains of transcription activation functions (AF): AF1 in NTD and AF2 inside LSD (Fig. 1). The oestrogen receptors ERα and ERβ are encoded by two separate genes located on chromosomes 6 and 14, respectively. ERα consists of 595 amino acid residues (67 kDa), and ERβ, depending on the isoform, is composed of a variable number of amino acid residues (53–59 kDa). Nuclear PgR is expressed in several isoforms that are products of a single PgR gene located on chromosome 11. The most represented isoforms are PgR -A (94 kDa) and PgR -B (120kDa) which are formed because of the initiation of transcription from two different promoters of the same gene. PgR-A is a truncated form of the receptor that lacks an N-terminal 164 amino acid residues, while PgR-B isoform is characterized by the presence of an additional AF3 in the NTD domain (Fig. 1).
Receptors of female sex hormones, oestrogen (ER) and progesterone (PgR), belong to the nuclear receptor (NR), which is a large superfamily of DNA-binding protein transcription factors. Nuclear ER and PgR regulate the transcription of specific genes by controlling the production of female sex hormones (oestrogens and progestins). Oestrogen and progesterone receptors have a similar structural and functional organization and are involved in controlling the development and functioning of the female reproductive system, involving the mammary gland, ovaries, and uterus, and are necessary for the ovulation process. The intracellular concentration of ER and PgR contributes to the regulation of the relationship between the parenchyma and stroma in the mammary gland. Normally, in the epithelium of the mammary gland, a low expression of ER and PgR is observed (7–30% of cells, depending on the cell cycle phase or hormonal background), while the expression of both markers is significantly increased during tumour transformation [13, 14].
Oestrogen and progesterone receptors determine the status of the primary breast tumour and are recognized as the most powerful prognostic markers of oestrogen- or progesterone- positive tumours controlled hormone therapy. Determination of steroid hormone receptors allows for making a prognosis of the disease. Patients initially diagnosed with breast cancer and negative ERs have a higher risk of recurrence compared to patients with a similar stage of the disease with positive ERs. In parallel, survival without recurrence and metastasis in patients whose tumours contain PgRs is significantly higher than in patients with tumours negative for PgRs. For patients of reproductive age, the expression of PgRs is less significant than for patients in menopause, but the expression of ERs is more significant [15-20].
3. THE MOST COMMONLY USED MONOCLONAL ANTIBODIES FOR MOLECULAR BIOLOGICAL MARKERS DETECTION
Achievements in fundamental immunology and modern biotechnology have made it possible to create a new generation of diagnostic drugs, such as monoclonal antibodies (mAbs). Kohler and Milstein [21] were the first to describe the production of hybridomas for immunodiagnostics capable of producing unlimited quantities of homogeneous populations of antibody molecules (Fig. 2). The advantages of mAbs are as follows: they are chemically pure reagents, identical in all parameters, capable of interacting with a unique antigen determinant, and available in unlimited quantities. Thus, using standard methods of working with cell cultures, it is possible to ensure an almost unlimited production. In the process of cloning and selection, a researcher can select hybridomas with desirable properties in terms of specificity of the interaction, affinity constants, and physicochemical properties that affect the possibility of their use in subsequent IHC analysis.
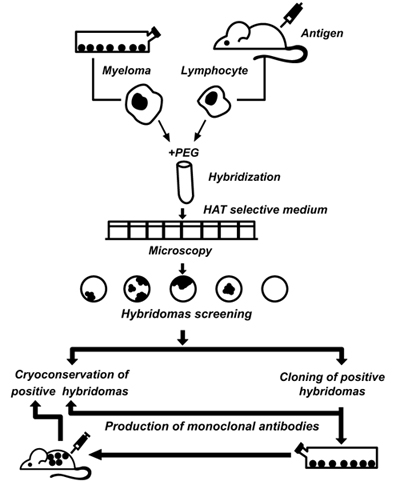
Currently, there are several hundreds of different mAbs that detect the expression of certain proteins associated with the structural components of the cell, receptors, and products of cell synthesis (hormones, enzymes, immunoglobulins). Modern test systems for tumour biomarkers are developed by leading pharmaceutical companies worldwide and constitute a respectable branch of the pharmaceutical industry.
The primary antibody imparts specificity to IHC and is used both as an antibody concentrate and as a ready-to-use (RTU) reagent. The use of RTU reagents has an important advantage; they allow the standardisation of reagents, dilutions, detection systems, and the use of a common protocol across laboratories. The most widely used mAbs for IHC diagnosis of breast cancer involving molecular markers HER2, Ki-67, ER, and PgR were determined in accordance with the Nordic Immunohistochemical Quality Control (NordiQC) assessments [22].
Analysis of the NordiQC protocols for quality control assessment of the expression level of the HER2 tumour marker from 2006 to 2020 shows that the FDA/IVD-approved assays are PATHWAY (Ventana), HercepTest (Dako), and Oracle (Leica) (Fig. 3A). The RTU system PATHWAY (Ventana), based on rmAb 4B5, is the leading system of laboratory use and has a stable efficiency (pass rate 95–100%). Since 2012, the HercepTest has lost its leading position and tended to decline in terms of laboratory use for assessing HER2 status. The polyclonality of the antibodies used in HercepTest (pAb SK001) causes sharp fluctuations in the pass rate for the investigated period. Furthermore, very low application parameters for HER2 detection use (less than 5% of the laboratories) and efficiency (pass rate 50–71%) are noted for mAb clone CB11 of Oracle.
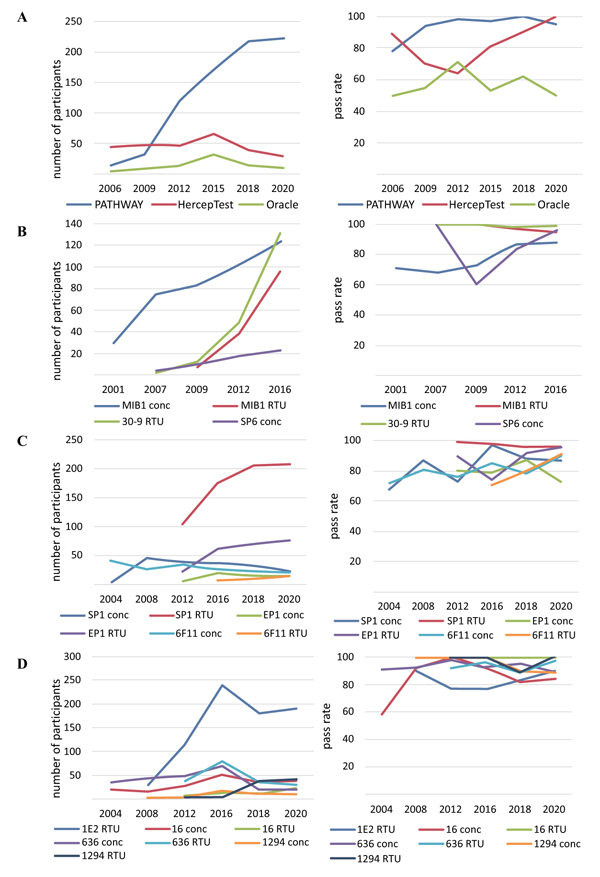
Quality control assessment of the expression level of the biomarker Ki-67 from 2001 to 2016 shows that the test systems based on the use of mAb MIB-1 (Dako) have existed for a long time and are most widely used in laboratories (Fig. 3B). The introduction of the RTU system mAb MIB-1 is characterized by a consistently high pass rate, with efficiency indicators reading 95–100%, compared to the same indicator for the concentrated format mAb MIB-1 (73–88%). To assess the Ki-67 status of patients, laboratories are also currently using the RTU system based on rabbit monoclonal antibodies 30-9 (Ventana), demonstrating the highest pass rate efficiency of 98–100%. The rabbit monoclonal antibody SP6 concentrated format turned out to be less popular for the detection of Ki-67, with less than 10% use in laboratories.
Assessment of the expression level of the ER biomarker from 2004 to 2020 indicates the dominant position of mAb SP1 (Ventana) in the RTU system (over 50% of the laboratories) and pass rate efficiency of 96–99% (Fig. 3C). For the detection of the ER biomarker, laboratories are actively using the RTU system mAb EP1 (Dako), with a tendency to increase the pass rate efficiency up to 95% in the latest tests. Less used by laboratories to assess ER status, mAb 6F11 (Leica) in RTU and concentrated formats and the concentrated formats of mAb SP1 and mAb EP1 are characterised by an average pass rate efficiency of 80–85%.
Assessment of the expression level of the PgR biomarker from 2004 to 2020 shows that most laboratories use mAb 1E2 (Ventana) in the RTU system (Fig. 3D). However, there are significant fluctuations in the efficiency of mAb 1E2, with a decrease in the pass rate to 77%, which is associated with the registration of false-positive results on negative control samples. According to the latest NordiQC 2020 test protocol, the efficiency of mAb 1E2 has again increased to 90%. Although the average efficiency pass rate of the remaining mAb 16 (Leica), 636, and 1294 (Dako) reached 90–95% over the indicated period, they are characterized by the use in laboratories at less than 10%.
The results on the most widely used mAbs for IHC diagnosis of breast cancer using molecular markers obtained from the NordiQC assessments are consistent with the test protocols of another independent organisation - UK NEQAS (United Kingdom National External Quality Assessment Service) [23]. The presented assessment of the diagnostic efficacy of mAbs to biomarker HER2, Ki-67, PR, and ER is supported by information and analysis of recent articles and reviews [3, 19, 20, 24-31].
4. THE EPITOPE SPECIFICITY OF MONOCLONAL ANTIBODIES FOR IMMUNOHISTOCHEMICAL DIAGNOSIS OF BREAST CANCER
The manufacturers of the most popular and effective mAbs for IHC diagnosis of breast cancer using molecular markers are the largest biopharmaceutical companies Ventana and Dako. In most cases, mAbs used are from rabbits rather than mice, as rabbits are believed to have a better immune response to small epitopes and tend to produce antibodies with higher affinity [32]. However, the effectiveness of antibodies in IHC is determined by the following major factors: affinity, epitope availability, and specificity. This fact is confirmed by an analysis of epitope specificity of mAbs for IHC diagnosis of breast cancer (Table 1).
| Clone | Manufacturer | Species | Clonality | Epitope |
|---|---|---|---|---|
| HER 2 | ||||
| 4B5 (PATHWAY) | Ventana | rabbit | mono | recognizes an epitope of the intracellular domain HER2 (amino acids region1242–1251) [24, 33] |
| pAbSK001 (HercepTest) | Dako | rabbit | poly | recognizes an epitope of the intracellular domain HER2 (amino acids region1244–1254) [24, 33] |
| CB11 (Oracle) | Leica | mouse | mono | recognizes an epitope of the intracellular domain HER2 (amino acids region1243–1249) [24, 33] |
| KI-67 | ||||
| MIB1 | Dako | mouse | mono | recognizes an epitope of the central domain Ki-67 (amino acid region 1160–1493) [25, 34, 35] |
| 30-9 | Ventana | rabbit | mono | recognizes an epitope of the C-terminus of Ki-67 (epitope unknown) [25, 36] |
| SP6 | Spring Bioscience | rabbit | mono | recognizes an epitope of the C-terminus of Ki-67(epitope unknown) [25, 37] |
| ER | ||||
| SP1 | Ventana | rabbit | mono | recognizes an epitope of the C-terminus ERα (amino acids region 578–595) [19, 30] |
| 6F11 | Leica | mouse | mono | recognizes an epitope of the N-terminus ERα (amino acids region 15–23) [19, 30] |
| EP1 | Dako | rabbit | mono | recognizes an epitope of the N-terminus ERα (amino acids region 37–42) [19, 30] |
| PgR | ||||
| 1E2 | Ventana | rabbit | mono | recognizes A and B isoforms of PgR (epitope unknown) [20, 31] |
| 16 | Leica | mouse | mono | recognizes the N-terminus A isoform of PgR (epitope unknown) [20, 31] |
| 636 | Dako | mouse | mono | recognizes an epitope of the N-terminus A and B isoforms of PgR (amino acids region 165–534) [20, 31] |
| 1294 | Dako | mouse | mono | recognizes an epitope of the N-terminus A and B isoforms of PgR (amino acids region165–534) [20, 31] |
For HER2 IHC detection, commercial mAbs, PATHWAY (Ventana), HercepTest (Dako), and Oracle (Leica), are reported to bind the intracellular domain near the C-terminal end of HER2 [24]. One of the unique features of the HER2 protein is that it undergoes in vivo proteolytic cleavage of its extracellular domain (ECD), generating a truncated 95-kDa intracellular protein (also called p95-HER2). When the ECD HER2 protein is lost, antibodies for HER2 can bind to the intracellular domain, producing positive IHC results [33]. The region in HER2 recognised by the three antibodies constitutes linear epitopes with minor shifts in the amino acid sequence.
The nuclear Ki-67 protein has an amphiphilic structure, its C-terminus can bind to DNA, and its N-terminus has an affinity for the cytoplasm. Although its splice variants differ in their N-terminus, they contain identical C-terminal and central (i.e., exon13) regions [9, 34]. These regions determine the epitope specificity of the most used mAb for Ki-67 detection in clinical practice: MIB-1, 30-9, and SP6 [25, 35-37].
Normally, mammary epithelial cells express 7–10% of ERα and 80–85% of ERβ, and level fluctuations in the former depend on the phase of the menstrual cycle. In tumour cells, the expression of ERα increases several times with a corresponding decrease in ERβ. In breast cancer, the study of the expression of ERs implies that Erα is an oncoprotein [14, 16, 18]. MAbs for ER detection recognise different epitopes of human ERα, 6F11 (Leica) and EP1 (Dako) recognise the N-terminus ERα, whereas SP1 (Ventana) directs the C-terminus ERα. There are differences in the sensitivity of these antibodies due to the C-terminus on the ERα molecule, which better preserves processing of antigen retrieval or fixation. Rabbit SP1 may have the highest affinity for the ERα receptor [19, 30, 38].
In humans, progesterone-producing cells express PgR-A and PgR-B at equivalent levels [15, 17]. Epitope mapping of the PR mAb 1E2, 16, and 636 clones showed that each antibody identified unique epitopes of the PgR molecule. Each clone binds to a different region of PgR, which is present within the PR isoforms [20, 31]. However, mAb clone 1E2 showed the highest affinity for PR in the binding kinetic analysis.
IHC analysis is based on the specificity of mAbs as the main immunoreagents, which enable the reliable identification of breast cancer cells. The discovery of the complete structure of these biomarkers and the design of their domains and subdomains by genetic engineering methods enable the synthesis of effective mAbs with epitope specificity.
CONCLUSION
The incidence of breast cancer is the highest worldwide, although its pathology is visually localised. Currently, it has been proven that the parameters of molecular genetic markers, such as oncoprotein HER2, proliferation markers Ki-67, oestrogen receptors ER, and progesterone receptors PgR, are associated with breast carcinogenesis and are a reflection of the biological aggression of the tumour.
IHC is a method used to determine the expression of biomarkers in tissues. IHC is a complex assay, where the result is influenced by multiple parameter optimisation and standardisation of all process stages, contributing to its reliability and reproducibility.
Today, due to the advances in hybridoma biotechnology, researchers and clinicians have their disposal preparations of mAbs that are standard and homogeneous agents available for production in laboratory conditions in unlimited quantities. The selection of the primary antibody has a significant impact on the IHC results, especially on diagnostic sensitivity and specificity. The effectiveness of antibodies in IHC is determined by the following major factors: affinity, epitope availability, and specificity. Quantitative indicators of the expression levels of tumor biomarkers of breast cancer, determined using mAbs, are an important indicator of the malignancy of cell tumorigenicity and are used to predict the course of the disease and select personalized therapy in clinical practice.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was funded by grant “Development of the method for determining the Ki-67 biomarker for assessing the level of cell proliferation in malignant tumors” (AP05133251) from the Ministry of Education and Science of the Republic of Kazakhstan.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


