All published articles of this journal are available on ScienceDirect.
A Comparative Proteomic Study of Thermobifida Cellulosilytica TB100T Secretome Grown on Carboxymethylcellulose and Rice Straw
Abstract
Background:
Cellulose, the major component of the plant cell wall, is the most abundant and cheap polymer on earth. It can be used by varieties of cellulolytic enzymes. Cellulases can hydrolyze cellulose to its glucose monomers, which can be fermented to many biotechnological products, such as biochemicals, bioplastics, and biofuels. Actinomycetes are potential sources of cellulases.
Objective:
The current study sheds light on the cellulolytic activity of Thermobifida cellulosilytica, a previously isolated thermophilic actinomycete, and the analysis of the lignocellulases produced in the secretome as a result of induction by different carbon sources.
Methods:
The cellulolytic activity was qualitatively confirmed by Congo red method showing a large halo zone around the colonies. The activity was also assayed using the 3,5-dinitrosalicylic acid (DNS) method. The secretome analysis was conducted by liquid chromatography-tandem mass spectroscopy (LC-MS/MS) based proteomic approach.
Results:
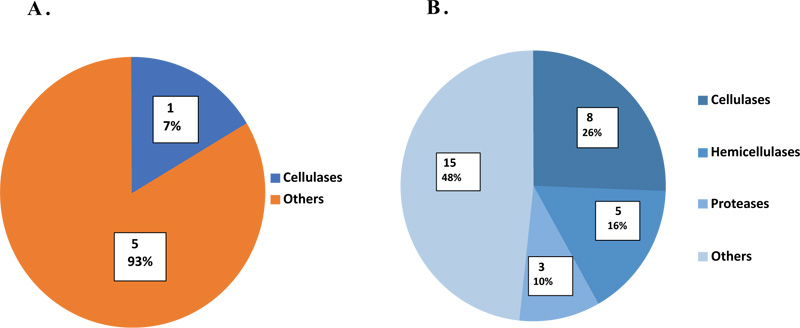
The cellulolytic activity increased by two folds upon the growth of T. cellulosilytica on rice straw (RS) as a complex substrate comparatively to Carboxymethylcellulose (CMC) as a simple one. These results were highly assured by LC-MS/MS. Where more proteins (n=31) were produced in the RS secretome, CMC produced only six proteins, including only one cellulase. Different classes of proteins produced in the RS secretome were cellulases (26%), hemicellulases (16%), proteases (10%), and others (48%).
Conclusion:
Lignocellulases are inducible enzymes. RS as a complex substrate induced T. cellulosilytica for the expression of more lignocellulolytic enzymes than CMC.
1. INTRODUCTION
The lignocellulosic materials, left behind agricultural and forestry industries, are mainly composed of cellulose 40-50%, hemicellulose 20-40%, and lignin 20-30% of plant dry weight [1]. Their production was estimated by 1.5 x 10 12 tons per year [2]. Cellulose is a linear polymer ranging from 10,000-15,000 glucose units linked by β-1,4 glycosidic bonds [3] and may reach up to 25,000 glucose units [4]. Due to its highly ordered crystalline structure, water insolubility as well as the presence of a tough lignin layer around it, cellulose is slowly/hardly degraded and unusable, leading to its disposition and accumulation as waste in nature [5]. This may lead to environmental pollution [6]. So, intervention to accelerate cellulose degradation and conversion to its fermentable sugars that can be used for biorefinery and industrial purposes is a must.
The efficient hydrolysis of cellulosic waste materials, has been employed either chemically or enzymatically [4, 7]. Chemical hydrolysis methods of cellulose degradation include incineration (the routine method), pyrolysis, steam explosion, and acidic or alkaline treatment [8]. Nowadays, application of these chemical methods has been dramatically limited and restricted only to be a preliminary/pretreatment step before enzymatic hydrolysis [9] due to their many disadvantages including environmental pollution, health problems, requirement of large equipment, and production of toxic chemicals or inhibitors as furfural or even undesirable non-sugar by-product [4, 5, 7].
On the other hand, the enzymatic degradation of cellulose can overcome all these drawbacks being clean, highly specific, and eco-friendly [7, 10]. The bioconversion of cellulosic wastes into its simple sugars has been employed by different hydrolytic and oxidative enzymes such as cellulases, hemicellulases (xylanases), lytic polysaccharide monooxygenases (LPMO) and peroxidases [1, 11]. Cellulases are a group of three enzymes; endoglucanases or CMCase (carboxymethylcellulases) (E.C. 3.2.1.4), exoglucanases or CBHs (cellobiohydrolases) (E.C. 3.2.1.91) and β-glucosidases (BGLs) or cellobiases (E.C. 3.2.1.21), acting synergistically for complete hydrolysis of cellulose [4]. Microbial lignocellulolytic enzymes are mainly produced from bacteria, fungi, and actinomycetes, the most effective secretors of lignocelluloses [12].
Thermobifida cellulosilytica TB100T strain is an actinomycete that has been isolated from manure compost region [13]. This strain showed 97.4% similarity to Thermobifida fusca, one of the well-characterized cellulolytic actinomycetes [13]. T. cellulosilytica becomes the subject of many recent researches due to its production of many important industrial enzymes such as cutinases and lignocellulases. Cutinases are efficiently used in plastic biodegradation, especially hydrolysis of PET (polyethylene terephthalate) [14]. Lignocellulases composed of cellulases, hemicellulases, and ligases are responsible for effective hydrolysis of lignocellulosic substrates [15].
Previous studies are focused on the identification of culture conditions, phenotypic, genotypic and taxonomic data of T. cellulosilytica TB100 T [ 13 ]. Little studies assessed the cellulolytic activity of this thermophilic strain [ 16 ]. Our research aimed to evaluate the cellulolytic potential of T. cellulosilytica grown on different carbon sources. This is the first work concerned with studying the effect of substrate type on the expression of different cellulases in the secretome collected from T. cellulosilytica using LC-MS/MS based proteomic analysis. This study can help the researchers interested in the production of thermophilic cellulases for industrial use.
2. MATERIALS AND METHODS
2.1. Bacteria and Culture Conditions
Thermobifida cellulosilytica TB100T (DSM 44535) used in the present study was ordered from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen), Germany. The lyophilized strain was aseptically resuspended in 1 ml of Czapek peptone broth containing: sucrose 30 g/L, NaNO3 3 g/L, K2HPO4 1 g/L, MgSO4.7H2O 0.5 g/L, KCl 0.5 g/L, FeSO4.7H2O 0.01 g/L, yeast extract 2 g/L, peptone 5 g/L and water to 1L. PH was adjusted to 7.3. A volume of 100 μL was added to 5 mL Czapek peptone broth for refreshment and was incubated at 45οC for 3-5 days. A glycerol stock was prepared and preserved at -80οC.
2.2. Qualitative Detection of The Cellulolytic Activity of T. cellulosilytica
The selected strain was cultivated on CMC agar media containing: CMC 0.5 g/L, NaNO3 0.1 g/L, K2HPO4 0.1 g/L, MgSO4 0.05 g/L, yeast extract 0.05 g/L, agar 20 g/L and water to 1L by spot inoculation method. The plates were incubated at 50οC for 72 h then flooded with 0.1% Congo red dye, gently shaken for 20 min, and excess dye was poured off. Finally, the plates were washed with 1M NaCl, gently shaken for 15 min and excess NaCl was poured off. The formation of a clear halo zone around the colony, that can be visualized as a purple color when flooded with 1 M HCl is considered as a positive result [17].
2.3. Effect of Different Substrates on Cellulase Production
Cellulase production was determined using CMC and RS, individually, as simple and complex substrate in the production medium containing the following constituents: CMC 5 g/L or pre-treated RS 1% (w/v), MgSO4.7H2O 0.2 g/L, KNO3 0.75 g/L, K2HPO4 0.5 g/L, FeSO4.7H2O 0.02 g/L, CaCl2 0.04 g/L, yeast extract 2 g/L and water to 1 L, pH was adjusted to 7.5. The RS was thermally and chemically pre-treated by NaOH. RS treatment was done as described by Taniguchi [18] as following: RS was cut into small pieces, washed with tap water for removal of surface dust, and soaked in 2.5% NaOH for 1h with a proportion of 1:10 (w/v). The mixture was autoclaved at 121οC for 30 min and then filtered. The residue was washed with distilled water several times till the supernatant pH became neutral. Finally, the residue was filtered again and dried in the oven at 80οC for 10 min. The microorganism was added to the flasks containing the previous production media with the corresponding substrates. The flasks were incubated at 50οC, shaken at 150 rpm for 11 days. A sample of one mL was periodically withdrawn every 24 h interval for assay of cellulase activity of T. cellulosilytica.
2.4. Cellulase Assay
After the specified incubation time, one milliliter of the production medium was withdrawn and centrifuged at 7000 xg for 10 min. Two hundred and fifty microliters of CFE (Cell Free Extract) was added to a reaction mixture composed of: 500 µL of CMC and 250 µL of 50 mM phosphate buffer, pH was adjusted to 7. The reaction mixture was incubated at 50οC for 2 h, and the resulted reducing sugars were quantified by the DNS method [19] using a microtiter plate reader (Tecan Sunrise, Switzerland). A standard curve was constructed based on at least six different glucose concentrations to estimate the reducing sugars released. One international unit of enzymatic activity was defined as the amount of enzyme that released 1 μmol of reducing sugar per min.
2.5. Partial Characterization of Crude Extract Produced from T. cellulosilytica
The CFE resulted from T. cellulosilytica growing on CMC as sole carbon source was used for characterization as following:
2.5.1. Optimum PH
Different buffer systems with different pH were prepared. One hundred mM Citrate buffer with pH 4.5, 5 and 5.5 and 100 mM phosphate buffer with pH 6, 6.5, 7, 7.5, 8 and 8.5 were used. The reaction mix was made with each prepared buffer and incubated at 50οC for 2 h. The enzyme activity was assayed by the DNS method.
2.5.2. Optimum Temperature
Five hundred microliters of CMC 0.5%, 250 μL buffer pH 8 and 250 μL supernatant were mixed in Eppendorf tubes. The tubes were incubated at different temperatures (4, 25, 30, 37, 50, and 70οC) for 2 h. The enzyme activity was assayed by the DNS method.
2.5.3. Optimum Substrate Concentration
A typical reaction was prepared as following: in Eppendorf tubes, 500 μL of different concentrations of CMC (0.1%, 0.25%, 0.5%, 0.75%, and 1%), 250 μL buffer pH 8 and 250 μL supernatant were mixed. The tubes were then incubated at 50οC for 2 h. A negative control tube containing distilled water instead of supernatant was incubated under the same conditions. The reducing sugar content was measured using the DNS method.
2.6. Secretome Analysis of T. cellulosilytica Grown on Different Cellulose Substrates
2.6.1. Strain Cultivation and Secretomic Protein Extraction
T. cellulosilytica TB100T strain was cultivated on the production medium containing the corresponding substrates (0.5% CMC and 1% RS, separately). The flasks were incubated at 50οC with shaking at 150 rpm for 7 days. The secretome from each substrate was harvested by centrifugation at 7000 xg at 4οC for 15 min twice to remove the bacterial pellets completely. The supernatant was concentrated and diafiltered through Vivaspin dialysis membrane with 5 kDa MWCO (GE Healthcare Life Sciences, Uppsala, Sweden) with 10 mM PO4-3 buffer pH 7, to one-tenth its starting volume. The protein concentration of each sample was determined using the bicinchoninic acid assay method (BCA). The resultant volume aliquots were preserved at -80οC.
2.6.2. Protein Digestion and LC-MS/MS Analysis
The secretory proteins from each sample were chemically denaturated by 8 M urea, reduced with 200 mM DTT (dithiothreitol) for 30 min at room temperature, alkylated with 1 M IAA (iodoacetamide) for 45 min to 1 h at room temperature in the dark and then enzymatically digested by trypsin containing procaine. The tryptic digestion was performed at 37 οC for 18 h (overnight) and then terminated by acidification with 100% FA (formic acid) to pH range 2-3 [20]. The resulting peptides were analyzed by Ekspert NanoLC425 HPLC system (Eksigent, USA) for chromatographic separation. Peptides (with 2 μL injection volume) from each sample were loaded onto trapping cartridge (10 x 0.5 mm) packed with CHROMXP C18CL 5μm particles, washed using mobile phase A (0.1% FA in DI-Water) at a flow rate of 10 μL/min for 3 min. Subsequently, peptides were fractionated on the analytical column (150 x 0.3 mm) packed with CHROMXP C18CL, 120 Aο, 3 μm particles. The mobile phase A and B (0.1% FA in acetonitrile) were run at a flow rate of 2 μl/min over 55 min to establish gradient elution that was 38 min 3-30% B; followed by 5 min 30-40% B and 2 min 40-80% B; maintained at 80% B for 3 min and finally equilibrated at 3% B.
Peptide fractions eluted from the HPLC system were directly injected into the mass spectrometer with nanospray® Ion Source. Data acquisition was set with a Triple TOF™ 5600+ system (AB SCIEX software), and the MS data were acquired in the positive ion mode, with a selected mass range of 400-1250 m/z. Smart IDA (Information Dependent Acquisition) was obtained through high-resolution TOF/MS survey scan followed by a product ion scan resulted from MS/MS analysis for the most abundant 40 ions whose threshold was over 150 counts, and charge ranged from +2 to +5 with cycle speed of 1.5 s.
2.6.3. Mass Spectrometric Data Analysis
Data acquisition was performed with Analyst® TF 1.7.1 (AB SCIEX software). MS raw data files from the Triple TOF™ 5600+ system were converted into MGF (Mascot Generic Format) files, which were then analyzed by Peptide Shaker (v1.16.27) for protein identification. The databases used for searching T. cellulosilytica TB100 were Swiss-prot and TrEMBL database containing 3,587 proteins [21]. The X!Tandem Algorithm was used for peptide identification. The data search parameters were set up as follows: Trypsin digestion with a maximum of two missed cleavage sites was considered along with fixed modification of cysteines by carbamidomethylation. Acetylation of lysine residues, peptide N termini, deamidation of asparagine and glutamine residues; and methionine oxidation were set as variable modifications. The presence of signal peptide sequences was determined using the ProtParam database [22].
2.6.4. Statistical Analysis
All experiments were done in at least two independent replicates, and the presented data are the mean values of replicates ± standard deviation using Microsoft Excel 2010 (Microsoft, USA). Differences between means were examined by one-way ANOVA (analysis of variance) followed by the Student’s t test using SPSS 22.0 software (SPSS Inc., Chicago, USA). The significance of differences was considered at a probability value less than 0.05.
3. RESULTS
3.1. Microorganism and its Cellulolytic Activity
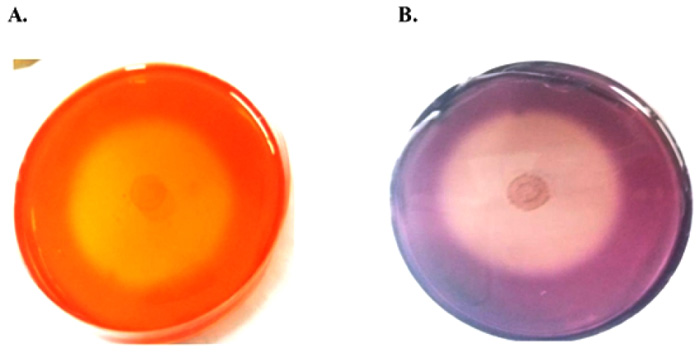
The cellulase activity of T. cellulosilytica was confirmed by Congo red method. It produced a wide clear halo zone around the colonies due to the degradation of CMC (Fig. 1).
3.2. Cellulolytic Activity Assay Using Different Carbon Sources
The cellulolytic activity of T. cellulosilytica TB100T was estimated upon its growth on either CMC or RS. The cellulase production was gradually increased till reaching its maximum activity that was estimated at 0.132± 0.080 IU/mL and 0.298±0.161 IU/mL on the 8th day and 7th day of incubation for both CMC and RS substrates, respectively. Subsequently, the cellulolytic activity decreased with longer incubation (Fig. 2).
3.3. Partial Characterization of Crude Extract
The cellulolytic activity of the CFE was optimal at pH 4.5 and 7.5 evaluated by 0.137 and 0.132 IU/mL, respectively, (Fig. 3A). The optimum temperature of cellulase was 50οC with a maximum activity of 0.136 IU/mL. Cellulase enzyme was able to tolerate temperature up to 70οC (Fig. 3B). The concentration of 0.75% of CMC was found to induce the highest amount of cellulase estimated by 0.132 IU/mL (Fig. 3C).
3.4. Secretome Analysis
The extracellular proteins of T. cellulosilytica TB100T, secreted in the presence of different substrates, were confidently identified by LC-MS/MS based proteomic analysis. After the enzymatic digestion of the secretome proteins by trypsin, a total of 9 peptides and 127 peptides were detected in the MS/MS spectra of CMC and RS, (Supplementary files 2 and 3). The resulted peptides were then searched against different databases giving 6 proteins and 31 proteins were produced in the CMC and RS, respectively, (Supplementary files 4 and 5).
The molecular weight and isoelectric point (PI) of all identified proteins were ranged from 15-106.3 kDa and 3.52-6.43 as shown in Tables 1 and 2. Most produced proteins had an acidic pH and extracellularly secreted by the action of signal peptides.
The secretomic produced proteins were functionally categorized and pie charted according to their roles in the deconstruction of lignocellulosic biomass (Fig. 4). Regarding CMC, the only secreted cellulolytic enzyme was glucanase (7%). On the other hand, the proteins detected in the RS secretome were grouped as cellulases (26%), hemicellulases (16%), proteases (10%), and other proteins (48%) as shown in (Fig. 4). Out of 31 proteins, 9 proteins (8.43) belonged to glycoside hydrolases (GHs) which were classified into different families according to (Carbohydrate Active enZYmes) CAZY database (http://www.cazy.org) [23]. The GH families included were GH6 (2), GH9 (2), GH10 (2), GH43 (1), GH48 (1) and GH81 (1). In addition, ten enzymes were annotated as containing carbohydrate binding modules (CBMs).
4. DISCUSSION
Actinomycetes are considered as potential sources of bioactive secondary metabolites like antibiotics, enzymes and growth factors [12]. Cellulose active enzymes, “cellulases” are considered as important enzymes certainly produced from actinobacteria, including T. cellulosilytica TB100T. An additional thermophilic property of T. cellulosilytica TB100T strain makes it a potential source for the production of thermostable cellulases, the cause of choice of the provided actinomycete strain in this study.
We confirmed the cellulolytic activity of T. cellulosilytica TB100T by Congo red which is the most widely used method for preliminary screening of cellulolytic actinomycetes, bacteria and fungi [5]. Congo red binds strongly with β-1,4-D glycoside linkage in polysaccharides, forming red complex [24]. This strong complex does not form except with polymers [25]. So, a large clear halo zone formed around colonies due to degradation of CMC polymer as a role of cellulase produced from T. cellulosilytica. Although the formation of a large clear zone on CMC agar plates, it doesn’t imply good productivity in liquid media [17].
It is also worth noting that the maximum CMCase activity of a thermophilic actinomycete, Thermomonospora fusca BD25 which is the closest one to the strain of our study, was also weak and estimated at 0.05 IU/mL, nearly half the CMCase activity from our strain of interest [26]. Similarly, lower weak cellulolytic activity was produced maximally from Geobacillus sp. DUSELR7 on day 7 and from Brevibacillus sp. DUSELG12 on day 10 as 0.058 IU/mL and 0.02 IU/mL respectively [27]. In another study, the cellulase activity of Bacillus subtilis AS3 was 0.07 IU/mL in basal control medium and increased 6 folds to be 0.43 IU/mL after cultivation in the optimized medium using Placket-Burman design [28].


A. Effect of different pH (▲) on cellulase production.
B. Effect of different incubation temperatures (♦) on cellulase production.
C. Effect of different CMC concentration (■) on cellulase production.
| Description/ Protein Name |
Uniprot
Accession NO. |
MW
(kDa) |
Coverage% | Peptides Matched | Amino Acid No. |
Protein
Family |
PI | CBM | Signal Peptide |
|---|---|---|---|---|---|---|---|---|---|
|
Cellulases Glucanase |
A0A147KIV9 |
61.9 |
2.38 |
2 |
588 |
GH6 |
4.30 |
CBM2 |
N |
|
Others Dihydrolipoyl dehydrogenase NADH dehydrogenase Glutamine synthetase Phospho-2-dehydro-3 deoxyheptonate aldolase Restriction endonuclease subunit R |
A0A147KD21 A0A147KF40 A0A147KFJ5 A0A147KJ47 A0A147KL42 |
48.3 50.0 53.2 50.8 117.4 |
14.16 0 0 0 0 0 |
7 1 1 1 1 |
459 459 474 468 1054 |
Pyr_redox_2 Pyr_redox_2 Gln-synt_C DHAP_synth_2 Helicase ATP-binding [HSDR_N]. |
5.44 9 5.01 6.11 5.60 |
- - - - - |
N N N N N |
| Description/ Protein Name | Uniprot Accession NO. |
MW (kDa) |
Coverage% | Peptides Matched | Amino Acid No. | Protein Family |
PI | CBM | Signal Peptide |
|---|---|---|---|---|---|---|---|---|---|
|
Cellulases Endoglucanase Cellulose 1,4-beta-cellobiosidase Glucanase Endoglucanase Glycoside Glycoside hydrolase Endoglucanase Endoglucanase Glucanase |
A0A147KLC2 A0A147KIK5 A0A147KI07 A0A0A7A855 A0A147KHU4 A0A147KHF1 A0A147KMV8 A0A147KIV9 |
68.3 106.3 46.6 46.2 100.8 103.8 102.4 61.9 |
5.02 15.9 17.4 4.78 1.59 16.17 17.45 34.69 |
2 16 11 3 1 14 15 17 |
618 981 454 439 941 965 957 588 |
Cellulase GH48 GH6 Cellulase GH81 GH9 GH9 GH6 |
4.51 4.35 5.77 4.71 5.03 4.67 4.14 4.30 |
CBM3 CBM2 CBM2 CBM2 - CBM2,3 CBM2 CBM2 |
Y Y N Y Y N N N |
|
Hemicellulases Beta-xylanase Beta-xylanase Xyloglucanase Xylose isomerase Beta-xylosidase |
A0A147KDP3 A0A147KJ27 A0A147KMS2 A0A147KE49 A0A147KMT4 |
53.4 50.5 97.8 43.1 61.8 |
11.02 21.1 2.29 3.12 2.18 |
8 8 5 1 3 |
490 474 919 385 550 |
GH10 GH10 - AP_endonuc_2 GH43 |
5.41 4.49 4.29 5.09 5.88 |
CBM2 CBM2 CBM2 - - |
Y Y Y N N |
|
Proteases Aminopeptidase Y Peptidase S1 ATP-dependent Clp protease ATP-binding subunit ClpX |
A0A147KFZ9 A0A147KH40 A0A147KHB4 |
53.8 38.2 46.9 |
8.74 2.95 3.3 |
6 1 1 |
515 373 424 |
Peptidase_M28 Peptidase S1 AAA |
5 4.48 5.03 |
- - - |
Y Y N |
|
Others Glutamyl-tRNA amidotransferase Uncharacterized protein Uncharacterized protein Peptide ABC transporter substrate-binding protein Growth inhibitor PemK Glycine/betaine ABC transporter substrate-binding protein Dihydrolipoyl dehydrogenase Surface protein Glutamate-binding protein Branched-chain amino acid ABC transporter substrate-binding protein Cell division protein DivIVA Cellulose-binding protein Protein GrpE 50S ribosomal protein L11 RNA helicase [Fragment] |
A0A147KH75 A0A147KDP0 A0A147KM68 A0A147KIK0 A0A147KJW7 A0A147KDQ0 A0A147KD21 A0A147KIW2 A0A147KMF2 A0A147KMP9 A0A147KL19 A0A147KKN7 A0A147KM11 A0A147KL98 A0A147KIV8 |
16.3 22.5 27.2 63.5 17.3 33.9 48.3 39.2 29.7 42.2 31.6 25.0 27.7 15.0 89.5 |
6.67 9.05 4.38 8.86 5.33 10.19 10.24 2.34 11.58 1.93 6.71 4.05 3.49 9.86 1.24 |
1 1 1 6 1 2 5 1 3 1 2 1 1 1 1 |
150 210 251 587 150 314 459 384 285 414 283 222 258 142 804 |
- - HTH GntR SBP_bac_5 PemK_toxin OpuAC Pyr_redox_2 TED SBP_bac_3 PBP6 DivIVA LPMO10 GrpE Ribosomal_L11 rRNA_proc_ arch |
6.43 11.15 6.02 4.08 5.37 4.02 5.44 4.34 4.06 3.52 4.96 8.40 4.32 9.10 9.02 |
- - - - - - - - - - - - - - - |
N N N Y N Y N Y Y N N Y N N N |
On the other hand, there are some actinobacterial strains in other studies that produced CM Case with moderate and high maximum activity including actinomycete BAY 21 with 1.381±0.024 IU/mL [7] and Streptomyces DSK59 with 20 IU/mL [12]. Another alkalothermophilic actinomycete produced CM Case with the activity of 23 IU/mL, 8.5 IU/mL and 12.5 IU/mL when cellulose paper powder, wheat bran and corncob were used as the substrate, respectively [29].
Regarding the cellulase, it is produced gradually till reaching maximum activity followed by a decline with longer incubation. Longer incubation period enhances the production of proteases. Proteases degrade subsequent enzymes resulting in diminished cellulolytic activity [30]. Prasad reported (2013) other reasons related to feedback inhibition occurred by the accumulation of end products [31].
Unlike most researches interested in the isolation of cellulolytic strains, the first study directed toward secretomic analysis of T. cellulosilytica TB100T was conducted [32]. The secretory protein profiles of T. cellulosilytica grown on different carbon sources, including CMC and RS, were compared. The proteomic study revealed a significant up-regulation in the lignocellulolytic enzymes expression of in the case of RS than CMC.
Lignocellulases are inducible enzymes [20]. Their production is dependent on the type or nature of the substrate used the culture media, which is known as substrate-induced phenomena [33, 34]. Do Vale and his colleagues (2012) showed that the complexity of the substrate affected the variety of the produced enzymes [35]. The CMC secretome contained six secretory proteins involving only one cellulase (glucanase). This low expression level of cellulases produced in the CMC secretome has an advantage of better isolation, purification, and studying the kinetic parameters of this cellulase, leading to decrease production cost on the large industrial scale.
On the contrary, more lignocelluloses (31 proteins) were identified in the RS secretome. The different classes of proteins produced were cellulases, hemicellulases, proteases, and other proteins. These varieties of enzymes were produced in the RS secretome due to its complex composition of cellulose (41%), hemicellulose (20%) tightly packed with lignin (12%) [36].
The lignin content of RS hinders the accessibility of the hydrolytic enzymes to their substrates [20]. Therefore, thermal and chemical pretreatment of RS is an essential process to make their cellulosic content more accessible to cellulases [37]. However, RS pretreatment is two-sided coin having drawbacks, including the formation of inhibitory products such as phenolics, aliphatic acids, and furan derivatives making feedback inhibition on the hydrolytic enzymes and further the fermentation process [38].
Most of the produced proteins contain carbohydrate-binding modules (CBMs) previously known as cellulose then carbohydrate binding domains (CBDs). CBMs are non-catalytic domains covalently bonded to the hydrolytic enzymes of different GH families. Like GHs, CBMs were classified into families ranged from family1 to 49 according to amino acid sequence similarities [39]. CBMs are responsible for protein-polysaccharide recognition [40]. They can target (hemi) cellulases to their carbohydrate polymers by increasing their concentrations at the substrates sites. Then, they change the carbohydrate structure, promoting their degradation by the effect of the hydrolytic enzymes [39, 41]. So, CBMs have a positive effect on increasing the rate and yield of the lignocellulolytic enzymes.
The absence of BGLs was noticed in the secretome of T. cellulosilytica grown on the corresponding substrates. This result was assured by quantitative assay for BGLs activity using pNPG (para nitro phenyl beta- D-glucopyranoside) as a substrate showing its negligible activity (data not shown). Our similar result was reported by other researches referring to the cause of the intracellular formation of this enzyme [42]. So, commercial cellulase preparations should be amended with BGLs from other sources, especially fungal ones, which are the highest BGL producers for efficient lignocellulolytic biomass hydrolysis [43].
The production, activity, and stability of actinomycetes, cellulases are greatly affected by pH and temperature [44]. Thermophilic cellulase system showed stability and activity at a wide pH range from 4.5-8.5 with a slight non-significant increase at pH 4.5 and 7.5 (p< 0.05). Other researches showed similar results in which the optimum pH range of cellulases was generally reported from 4.2-5.2 [45]. However, Prasad and co-workers (2013) assured that maximum cellulase activity from Streptomyces griseorubens (Accession No. AB184139) was at pH 7 [31]. The activity of CM Case from Bacillus sp. SMIA-2 was found to be optimum at pH 8 [46]. The main reason may be attributed to the complex cellulase system required for complete cellulose degradation. This complex system is composed of 3 different enzymes acting synergistically [47]. Each enzyme has its optimum pH that ensures proper working.
Cellulase isolated from T. cellulosilytica showed the maximum activity at 50οC with 94% of retained activity at 70οC. Other researchers showed similar results from Streptomyces lividans66, Bacillus sp., MSL2 strain, Bacillus sp DUSELR13, and Geobacillus sp WSUCF1 [48, 49]. This higher temperature reaction leads to faster reaction rates and better degradation of cellulosic materials [46]. The maximum cellulolytic activity achieved at 0.75% concentration of CMC. Higher concentrations were too viscous that hindered the cellulolytic assay.
CONCLUSION
T. cellulosilytic a is a promising source of thermostable cellulases that are active over a wide range of pH and temperature. Also, LC-MS/MS based proteomic analysis revealed that T. cellulosilytic a has variable cellulases that are efficient for the degradation of simple/complex lignocellulosic materials. T. cellulosilytic a is an interesting actinomycete for more studies in the production of thermostable cellulases for successful applications, such as biofuel production.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are thankful for the proteomics unit in the children’s cancer hospital, especially Dr. Sameh Magdeldin, Dr. Hassan Shikshaky, and Dr. Aya Osama for the proteomic analysis. We would like to thank Dr. Haitham Saeed for supporting this study.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
Supplementary file 1: Pellets of T. cellulosilytica grown on 0.5% CMC and 1% RS after 2 days and 7 days of incubation.
Supplementary file 2: Peptides identified in the CMC secretome by LC-MS/MS technique.
Supplementary file 3: Proteins identified in the CMC secretome by LC-MS/MS technique.
Supplementary file 4: Peptides identified in the RS secretome by LC-MS/MS technique.
Supplementary file 5: Proteins identified in the RS secretome by LC-MS/MS technique.


