All published articles of this journal are available on ScienceDirect.
Chemical Profiling and Antimicrobial Properties of Phyto-Active Extracts from Terminalia glaucescens Stem Against Water Microbial Contaminants
Abstract
Background:
The present study was designed to evaluate the phytochemicals of Terminalia glaucescens stem extracts and test their antimicrobial potency against water microbial contaminants reported to be multidrug resistant.
Method:
Dry stem powder was extracted with ethanol, ethyl acetate and chloroform. These fractions were then examined for antimicrobial activity by using disc diffusion assay against typical clinical bacteria and fungal isolates which have been reported as water contaminants. The microbial strains were exposed to five different concentrations of extracts: 500 mg/ml, 250 mg/ml, 100 mg/ml, 50 mg/ml and 25 mg/ml.
Result:
It was observed in this study that increase in the concentration of extracts correlated with microbial growth inhibition. In-vitro phytochemical screening of plant extracts revealed the presence of alkaloid, flavonoid, saponin, terpenoid, steroid and anthraquinones. Ethanolic extract performs better than ethyl acetate and chloroform extracts, as it recorded the highest zone of inhibition of 20.5 mm against Streptococcus pneumoniae while ethyl acetate and chloroform recorded 17.50 mm each against Streptococcus pneumoniae and Bacillus cereus, respectively. Ethanolic extract also showed the highest antifungal activity against Trichoderma sp. and Aspergillus niger. The antibacterial and antifungal activities of active extracts were observed in the increasing order Ethanol>Chloroform≥ethyl acetate with respect to the maximum zone of inhibition. Activity of crude extract from ethanol, when further compared with commercial antibiotics (Gentamicin, Streptomycin and Nystatin), was significantly higher.
Conclusion:
This plant crude extracts could therefore serve as potential source of new biocides with application in water research and other biotechnological fields.
1. INTRODUCTION
Fungi and bacteria are of veterinary and human significance. Bacillus cereus has been involved in foodborne intoxication [1]. Escherichia coli and Pseudomonas aeruginosa cause ailments like mastitis, abortions and upper respiratory complications. Streptococcus spp is a pathogenic bacteria usually found in the intestines of birds [1, 2]. Fungi like Aspergillus niger, have been reported to cause lung diseases, aspergillosis and otomycosis. Aspergillus flavus is a livestock and human pathogen associated with aspergillosis of the lungs and sometimes causes corneal, otomycotic and naso-orbital infections. They produce significant quantity of aflatoxin [3, 4]. Candida albicans is reported to cause asthma [5], vaginitis and yeast mastitis. These sets of organisms have been described to be multi-drug resistant and established as recent contaminants of water bodies [6-10]. Research in recent years have revealed multidrug resistance in different bacterial species [6, 8]. Some emerging fungal water contaminants have also been reported [7]. Some of these resistant microbial spp are currently being found in water bodies [9,10]. Reports exist to show the continuous isolation of pathogenic microorganisms especially, antibiotic resistant strains from water and wastewater. Sisti [11] and Sidhu [12], reported a high incidence of bacteria spp from influent and effluent of urban waste and water purification plants. In addition, bacteria spp isolated from sewage have also been found in fresh water and drinking water even after chlorination. Health and environmental implications of these organisms, necessitate a search for novel lead compounds that could be used against such microbes. The pitfalls of chemical coagulants and disinfectants in water such as alum and chlorine also necessitate studies to further develop biomaterials as green and low cost treatment alternative for global treatment and management amidst growing global water crises [5]. It is also incumbent to curb and treat this water contamination bearing in mind that the total water volume on earth cannot be increased. Interest in plants with antimicrobial properties has been revitalized as a consequence of antimicrobial resistance attributed to indiscriminate use of commercial drugs [13], undesirable side effects of synthetic antimicrobials with respect to the emergence of previously uncommon diseases [14, 15], limited effective life span and high cost of synthetic antimicrobials [16]. The efficacy of synthetic antimicrobial agents has been abridged due to the continuing emergence of drug resistant organisms and the adaptations by microbial pathogens to commonly used antimicrobials [17]. While hundreds of plant species had been tested for antimicrobial properties, the vast majority of plants remained unassessed [18-20]. In spite of the presence of numerous approaches to drug discovery, plants still remain the leading reservoir of natural medicines [21] and represent a starting point for antimicrobial compounds discovery [22].
Terminalia glaucescens is a key component of the plants used in the formulation of the “wonder cure” concoction used in the management of tuberculosis in Nigeria. The efficacy of the plant extract on Mycobacterium tuberculosis was established by Adeleye et al. [23]. Terminalia species are used as traditional medication in the treatment of dysentery, and also found use in the last stages of management of AIDS [24, 25]. It is anticipated that plant secondary metabolic extracts with target sites other than those used by antibiotics will be effective against drug resistant pathogens [26]. These extracts are of great importance to scientists working on infectious diseases because they signify a possible source of unique antibiotic prototypes [6, 16]. Approximately 80% of the populaces in developing nations use traditional medical specialties for their wellness and care [27]. The genus Terminalia is the second largest genus of the Combretaceae after Combretum, with about 200 species. These plants spread in tropical areas of the globe with the greatest genetic diversity in Southeast Asia [28]. Genus Terminalia gets its name from Latin terminus, as the leaves appear at the tips of the shoots [29]. Terminalia species range from shrubs to huge deciduous forest trees. Mostly they are very large trees reaching in height up to 75 m tall [30]. Terminalia spp are widely used in traditional medicine in a number of continents in the world for the treatment of diseases including, abdominal disorders, bacterial infections, colds, diarrhea, dysentery, sore throats, conjunctivitis, fever, heart diseases, hookworm, gastric ulcers, headaches, hypertension, jaundice, edema, pneumonia, leprosy, nosebleed, and skin diseases [31]. The fruits of both T. chebula and T. bellerica are vital constituents of “triphala”, a popular Ayurvedic formulation that possesses several activities in the Indian traditional medicine [32]. T. chebula fruit possesses an amazing power of healing and is called the “King of Medicine” in Tibet as it is used for the treatment of various illnesses [33, 34]. The Bark of T. arjuna is used as cardioprotective and anti-hyperlipidemia in folklore treatment [35]. In Africa, T. mollis is used to treat gonorrhea, malaria, diarrhea, and HIV management, while T. brachystemma is used for the treatment of shistosomiasis and gastrointestinal complaints [36].
Phytochemicals, are often secondary metabolites existing in smaller amounts in higher plants which include terpenoids, tannins, alkaloids, steroids, flavonoids and many others [37]. Different studies and researchers have used different extraction procedures and investigated medicinal importance in several plants. Tom et al. [38] and others [39-42] have equally investigated the medicinal importance of different Terminalia spp using different extraction methods. A current expectation in this field of inquiry is the hunt for emerging plants and plant materials with novel biotechnological applications such as the application of Cola nitida and D. eriocarpum in water purification and nanoparticle synthesis as paint additives [43, 44]. Table 1 consists of some biotechnological applications of plant extracts.
| Application areas | Plant | Reference |
|---|---|---|
| Bio-fertilizers | Tea-seed (Camellia sp) Ascophyllum nodusum |
Andresen and Cedergreen, 2010; Norrie and Keathley, 2006. [70, 71] |
| Artificial insemination |
Curcurbita pepo Khaya senegalensis |
Yongabi, 2005 [72] |
| Bio-shampoo | Accacia concinna (Willd.) DC, Averrhoa bilimbi Linn. and Tamarindus indica Linn.) | Rassami and Soonwera, 2013 [73] |
| Drink flavours | Tetrapleura tetraptera | Korankye, 2010 [74] |
| Food colourant | Tefashia sp | Korankye, 2010 [74] |
| Fumigants | Syzygium aromaticum | Rahuman et al., 2011 [75] |
| Insect repellants, larvicides insecticides and pesticides |
Mesua ferra L, Tephrosia vogeli, Petivera alliacea. | Olaitan and Abiodun, 2011; Singha et al., 2011 [76, 77] |
| Livestock ethno-therapy |
Carica papaya Zingiber officinale |
Yongabi, 2005 [72] |
| Nanoparticle production and paint additives | Cola nitida | Lateef et al. 2015; Lateef and Adeeyo, 2015 [43, 46] |
| Water purification | Moringa oleifera, Jatropha curcas, Guar gum and Dicerocaryum eriocarpum | Pritchard et al., 2009 Odiyo et al., 2017 [44, 78] |
The present study reports the phytochemical screening and antimicrobial activities of Terminalia glaucescens extracts as potential biocide against emerging microbial water contaminants.
2. MATERIALS AND METHODS
2.1. Materials
The chemicals used were obtained from Sigma–Aldrich, Germany through Lab Trade Chemicals Limited Nigeria and are of Analytical grades. Filter papers were from Whatman, GE Healthcare companies, China. The bacterial strains were obtained from Ladoke Akintola Univerity of Technology (LAUTECH) Teaching Hospital, and Obafemi Awolowo Univeristy (OAU), Nigeria.
2.2. Plant Sample
Newly harvested Terminalia glaucescens were obtained from Oja Igbo market, Ogbomoso North local government, Ogbomoso, Oyo State, Nigeria. Plant samples were moved to the lab immediately in polyethylene bags for preparation in order to avoid the decomposition of bioactive compounds.
2.3. Preparation of Samples
The plant stems were carefully washed in order to get rid of contaminants and impurities and were, shredded into smaller pieces, dried at 25±2 OC, ground into powder, filtered into fine particles and then extracted.
2.4. Extraction Procedure for Dry Leaf Powder Samples
50 g of dried and powdered Terminalia glaucescens stem was extracted in 150 ml of chloroform, ethanol and ethyl acetate for a duration of 48 hrs. The solvents were separated using sterile muslin cloth and sieved through sterile Whatmann filter paper (No. 01). The resultant extract in solvents was evaporated to dryness in a rotary evaporator and used as crude extract. The dry crude extracts were used for antimicrobial and phytochemical studies.
2.5. Preliminary Phytochemicals Screening
The phytochemical screening of the extracts was investigated according to the methods of Harborne [45] with little modifications as follows;
2.5.1. Saponins
The presence of saponin was detected by froth test. Exactly 500 mg of extract was measured into a 250 ml conical flask with 25 ml of sterile distilled water and heated for 5 minutes. This was then sieved and after which 2.5 ml of the filtrate was added to 10 ml of sterile distilled water in a test tube. The mixture was then vigorously shaken for about 30 seconds and left for 30 minutes. Honeycomb froth showed the presence of saponins.
2.5.2. Alkaloids
Alkaloid test was carried out by measuring 2 ml of chloroform into a test tube to which few drops of Wagners reagent and 1 ml of the crude filtrate solution were added. A reddish brown precipitate formation indicated the presence of alkaloids.
2.5.3. Tannins
To investigate the presence of tannins, to a portion of crude filtrate solution in distilled water, about 4 drops of 20% ferric chloride solution were carefully added. The formation of green, blue or blue-black colour indicated the presence of tannins.
2.5.4. Reducing Sugars
To 0.5 ml of extracts 1 ml of water and few drops of Fehling’s solution were added. The mixture was heated over water-bath and the formation of brick red precipitate confirmed the presence of reducing sugars.
2.5.5. Terpenoids
The presence of terpenoids was confirmed by adding 1 ml of crude extract solution to 2 ml chloroform followed by the addition of acetic anhydride (1 ml). One milliliter of concentrated sulphuric acid was carefully introduced to the solution. The formation of red violet colour mixture showed the presence of terpenoids.
2.5.6. Flavonoids
Few drops of diluted sodium hydroxide solution was added to 0.5 ml extract. A deep yellow colour mixture which became colourless upon the addition of few drops of diluted H2SO4 acid proved the presence of flavonoids.
2.5.7. Steroids
About 0.5 ml of acetic anhydride and few drops of concentrated H2SO4 were added to 1 ml of extract filtrate. A blue-green precipitate shows the presence of steroids.
2.6. Antimicrobial Activity Study
Extracts preparation: Crude extract of the T. glaucescens, was prepared as different concentrations in different solvents through serial dilution in appropriate solvents (ethanol, ethyl acetate and chloroform) to give (500 mg/ml, 250 mg/ml, 100 mg/ml, 50 mg/ml and 25 mg/ml designated as C5, C4, C3, C2 and C1, respectively) and was used for the antimicrobial screening. The antimicrobial potency test was carried out using the agar disc diffusion method [46]. Whatman No 1 filter paper was used in the preparation of 6.0 mm diameter discs embedded with extracts at various concentrations and used in the sensitivity test. Negative controls (C) were prepared by using the same solvents employed to dissolve the samples. Inhibition zones were measured and compared with the standard synthetic antibiotics as reference.
Antimicrobial Sensitivity Test: Test organism was swabbed evenly on the surface of the agar plate using sterile swab sticks. Impregnated paper discs containing the plant extract at different concentrations were then arranged radially and pressed slightly and firmly on the inoculated agar surface to ensure even contact. The plates were then incubated at 37 oC for 24 hours on Nutrient Agar (for bacteria isolates) while the fungal plates (on Potato Dextrose Agar) were incubated at 25 ±2 oC for 48 hrs. The level of sensitivity was defined by assessing the diameter (in millimeter) of the visible zone of inhibition of microbial growth produced by the dispersion of the infusion. Each method in this experiment was repeated three times.
Test organisms: The bacterial isolates used in this study include Klebsiella spp, E.coli, Pseudomonas auriginosa, Streptococcus pneumoniae and Bacillus aureus. While the fungal isolates were Aspergillus flavus, Aspergillus niger, Trichoderma spp and Candida spp, and the cultures were maintained on nutrient agar and potato dextrose agar, respectively.
Statistical Analysis: The Statistical Package for Social Scientists (SPSS, version 19.0) was used for the analysis of the data obtained. Two way ANOVA test was applied to set the degree of significance of the crude extracts at different concentrations. Also, comparison of the different solvent extractions (i.e. ethanol, ethyl acetate and chloroform) were made statistically; the general antimicrobial effects of the extracts were compared with the standard antibiotics and antifungal disc, respectively.
3. RESULTS
The results obtained from the antimicrobial activity of T. glaucescens on the test organisms are presented in Tables 2 to 5. The data were analyzed statistically and the significance level was obtained at p<0.05. Values are shown as mean ± standard deviation (SD) for each immersion. Values with (*) are significantly higher than the control at p < 0.05.
| Zones of inhibition of different extracts | |||||||
|---|---|---|---|---|---|---|---|
| Ethanol (mm) | Ethyl Acetate(mm) | Chloroform(mm) | |||||
| P. aeruginosa | C | 8.50 | ± 0.707 | 7.50 | ± 0.707 | 7.00 | ±0.000 |
| C1 | 9.50 | ± 0.707 | 8.50 | ± 0.707 | 7.50 | ± 0.707 | |
| C2 | 11.50 | ± 0.707* | 9.00 | ± 1.414 | 8.50 | ± 0.707 | |
| C3 | 11.50 | ± 0.707* | 11.50 | ± 0.707* | 8.50 | ± 0.707 | |
| C4 | 13.50 | ± 0.707* | 12.00 | ± 1.414* | 9.50 | ± 0.707 | |
| C5 | 14.50 | ± 0.707* | 13.50 | ± 0.707* | 10.50 | ± 0.707* | |
| Klebsiella sp | C | 9.00 | ±1.414 | 7.50 | ± 0.707 | 7.00 | ±0.000 |
| C1 | 8.50 | ±2.121 | 7.50 | ± 0.707 | 7.50 | ± 0.707 | |
| C2 | 9.50 | ± 0.707 | 8.50 | ± 0.707 | 8.00 | ±0.000 | |
| C3 | 11.50 | ± 0.707 | 10.00 | ±0.000 | 8.50 | ± 0.707 | |
| C4 | 12.00 | ±1.414* | 10.50 | ± 0.707* | 8.50 | ± 0.707 | |
| C5 | 13.50 | ± 0.707* | 11.50 | ± 0.707* | 10.00 | ±0.000* | |
| E. coli | C | 8.50 | ± 0.707 | 7.00 | ±0.000 | 7.50 | ± 0.707 |
| C1 | 10.50 | ± 0.707 | 8.50 | ± 0.707 | 8.00 | ±01.414 | |
| C2 | 14.00 | ±1.414* | 9.50 | ± 0.707 | 8.50 | ± 0.707 | |
| C3 | 15.00 | ±1.414* | 11.00 | ±0.000* | 9.50 | ± 0.707 | |
| C4 | 18.00 | ±1.414* | 12.50 | ± 0.707* | 11.50 | ± 0.707 | |
| C5 | 20.00 | ±1.414* | 13.00 | ±1.414* | 13.50 | ±02.121* | |
| S. pneumonia | C | 8.50 | ± 0.707 | 7.00 | ±0.000 | 7.50 | ± 0.707 |
| C1 | 12.00 | ±1.414 | 8.50 | ± 0.707 | 8.00 | ±0.000 | |
| C2 | 14.00 | ±1.414* | 9.50 | ± 0.707 | 7.50 | ± 0.707 | |
| C3 | 17.00 | ±1.414* | 12.00 | ±1.414* | 8.50 | ± 0.707 | |
| C4 | 19.00 | ±0.000* | 14.50 | ± 0.707* | 10.50 | ± 0.707* | |
| C5 | 20.50 | ± 0.707* | 17.50 | ± 0.707* | 13.50 | ± 0.707* | |
| B. cereus | C | 8.50 | ± 0.707 | 7.00 | ±0.000 | 8.00 | ±0.000 |
| C1 | 12.00 | ±1.414 | 9.00 | ±1.414 | 10.50 | ± 0.707 | |
| C2 | 15.00 | ±1.414* | 12.50 | ± 0.707* | 13.00 | ±1.414* | |
| C3 | 16.00 | ±1.414* | 13.50 | ± 0.707* | 14.00 | ±1.414* | |
| C4 | 18.50 | ± 0.707* | 13.50 | ±2.121* | 16.00 | ±1.414* | |
| C5 | 19.00 | ±1.414* | 16.00 | ±1.414* | 17.50 | ± 0.707* | |
| Zones of inhibition of different extracts | |||||||
|---|---|---|---|---|---|---|---|
| Ethanol (mm) | Ethyl Acetate(mm) | Chloroform(mm) | |||||
| A. flavus | C | 8.50 | ± 0.707 | 7.50 | ± 0.707 | 7.00 | ±0.000 |
| C1 | 12.00 | ±2.828 | 9.50 | ± 0.707 | 9.50 | ±2.121 | |
| C2 | 13.50 | ±2.121 | 12.00 | ±1.414* | 10.50 | ± 0.707 | |
| C3 | 17.00 | ±1.414* | 13.00 | ±1.414* | 14.50 | ± 2.121* | |
| C4 | 17.50 | ± 0.707* | 14.50 | ± 0.707* | 15.50 | ± 2.121* | |
| C5 | 19.00 | ±1.414* | 15.50 | ± 0.707* | 17.00 | ± 1.414* | |
| A. niger | C | 7.50 | ± 0.707 | 7.00 | ± 0.000 | 7.00 | ± 0.000 |
| C1 | 11.00 | ±1.414 | 8.50 | ± 0.707 | 10.00 | ± 1.414 | |
| C2 | 14.00 | ±1.414* | 11.00 | ± 1.414* | 13.00 | ± 1.414* | |
| C3 | 17.50 | ± 0.707* | 13.50 | ± 0.707* | 15.50 | ± 0.707* | |
| C4 | 18.50 | ± 0.707* | 13.00 | ± 1.414* | 16.50 | ± 0.707* | |
| C5 | 19.00 | ± 0.000* | 15.50 | ± 0.707* | 17.50 | ± 0.707* | |
| Trichoderma sp | C | 8.50 | ± 0.707 | 7.00 | ± 0.000 | 7.50 | ± 0.707 |
| C1 | 9.00 | ± 1.414 | 8.50 | ± 0.707 | 9.50 | ± 0.707 | |
| C2 | 10.00 | ± 1.414 | 8.00 | ± 1.414 | 9.00 | ± 1.414 | |
| C3 | 11.00 | ± 1.414 | 8.50 | ± 0.707 | 10.00 | ± 1.414 | |
| C4 | 14.00 | ± 1.414* | 11.00 | ± 1.414* | 12.50 | ± 0.707* | |
| C5 | 19.00 | ± 1.414* | 12.50 | ± 0.707* | 16.00 | ± 1.414* | |
| Candida sp | C | 8.00 | ± 1.414 | C | ± 0.000 | 7.50 | ± 0.707 |
| C1 | 11.00 | ± 1.414 | 9.00 | ± 1.414 | 10.00 | ± 1.414 | |
| C2 | 13.00 | ± 1.414 | 9.50 | ± 0.707 | 11.00 | ± 1.414 | |
| C3 | 12.00 | ± 1.414 | 10.00 | ± 1.414 | 11.00 | ± 1.414 | |
| C4 | 13.50 | ± 0.707* | 12.00 | ± 0.000* | 11.00 | ± 0.000 | |
| C5 | 15.00 | ± 1.414* | 12.00 | ± 1.414* | 13.00 | ± 1.414* | |
| Bacteria | Highest zone of inhibition (mm) |
|---|---|
| Ethanol | 20.5 |
| Ethyl acetate | 17.5 |
| Chloroform | 17.5 |
| Fungi | |
| Ethanol | 19.0 |
| Ethyl acetate | 15.5 |
| Chloroform | 17.5 |
| Ethanol | |||
|---|---|---|---|
| P. aeruginosa | STR | 11.50 | ± 0.707 |
| C1 | 9.50 | ± 0.707 | |
| C2 | 11.50 | ± 0.707 | |
| C3 | 11.50 | ± 0.707 | |
| C4 | 13.50 | ± 0.707 | |
| C5 | 14.50 | ± 0.707* | |
| Klebsiella sp | STR | 10.50 | ± 0.707 |
| C1 | 8.50 | ±2.121 | |
| C2 | 9.50 | ± 0.707 | |
| C3 | 11.50 | ± 0.707 | |
| C4 | 12.00 | ± 1.414 | |
| C5 | 13.50 | ± 0.707 | |
| E.coli | STR | 10.00 | ± 0.000 |
| C1 | 10.50 | ± 0.707 | |
| C2 | 14.00 | ± 1.414 | |
| C3 | 15.00 | ± 1.414* | |
| C4 | 18.00 | ± 1.414* | |
| C5 | 20.00 | ± 1.414* | |
| S. pneumonia | GEN | 13.50 | ± 0.707 |
| C1 | 12.00 | ± 1.414 | |
| C2 | 14.00 | ± 1.414 | |
| C3 | 17.00 | ± 1.414 | |
| C4 | 19.00 | ± 0.000 | |
| C5 | 20.50 | ± 0.707* | |
| B. cereus | GEN | 13.00 | ± 0.000 |
| C1 | 12.00 | ± 1.414 | |
| C2 | 15.00 | ± 1.414 | |
| C3 | 16.00 | ± 1.414 | |
| C4 | 18.50 | ± 0.707* | |
| C5 | 19.00 | ± 1.414* | |
| A. flavus | NIS | 15.00 | ± 0.000 |
| C1 | 12.00 | ± 2.828 | |
| C2 | 13.50 | ± 2.121 | |
| C3 | 17.00 | ± 1.414 | |
| C4 | 17.50 | ± 0.707 | |
| C5 | 19.00 | ± 1.414 | |
| A. niger | NIS | 16.50 | ± 0.707 |
| C1 | 11.00 | ± 1.414 | |
| C2 | 14.00 | ± 1.414 | |
| C3 | 17.50 | ± 0.707 | |
| C4 | 18.50 | ± 0.707 | |
| C5 | 19.00 | ± 0.000 | |
| Trichoderma sp | NIS | 14.00 | ± 0.000 |
| C1 | 9.00 | ± 1.414 | |
| C2 | 10.00 | ± 1.414 | |
| C3 | 11.00 | ± 1.414 | |
| C4 | 14.00 | ± 1.414 | |
| C5 | 19.00 | ± 1.414 | |
| Candida sp | NIS | 14.00 | ± 0.000 |
| C1 | 11.00 | ± 1.414 | |
| C2 | 13.00 | ± 1.414 | |
| C3 | 12.00 | ± 1.414 | |
| C4 | 13.50 | ± 0.707 | |
| C5 | 15.00 | ± 1.414 | |
3.1. Antimicrobial Effect of Crude Extracts of Terminalia glaucescens
The antimicrobial activities of ethanol, chloroform and ethyl acetate extract of T. glaucescens with respect to their zones of inhibition (mm) on the test organisms are shown in Tables 2 to 4. All the extracts showed potential antimicrobial activities against the test organisms. The antimicrobial activity of the extracts increased with increase in extract concentrations.
Ethanolic extract: Ethanolic extract (C1 – C5) effectively inhibited majority of the test organisms and was comparatively better in antimicrobial performance than the control. The zone of inhibition against test organisms ranged from 8.5 mm at C1 – to 20.5 mm at C5. The extract shows inhibition against test fungi with zones of inhibition ranging from 9.0 mm at C1 – 19.0 mm at C5 while zones of inhibition against bacteria isolates tested ranged between 8.50 mm at C1 - 20.5 mm at C5. Ethanolic extract at concentration C5 (500 mg/ml) showed consistently higher zones of inhibition against S. pneumonia (20.5 mm), E.coli (20.0 mm), B. cereus, A. niger, Trichoderma sp. and A. flavus (19.0 mm) respectively, while the lowest zone of inhibition at this concentration was recorded against Klebsiella sp with a diameter of 13.5 mm which is, however, higher than the performance of the control with a record range of 8.50 mm – 9.00 mm.
Ethyl acetate extract: Ethyl acetate extract like ethanolic extract effectively inhibited the growth of all tested bacteria and fungi and performed better than the control except for activity against Klebsiella sp where the performances were comparatively the same with the control and recorded as 7.50 mm. The range of inhibition of the extract was from 7.5 mm at C1 – 17.5 mm at C5. Ethyl acetate extract recorded zone of inhibition against test fungal isolates in the range of 8.0 mm at C2 – 15.5 mm at C5 while zones of inhibition against bacteria isolates tested ranged from 7.50 mm at C1 - 17.5 mm at C5. The highest zone of inhibition recorded for ethyl acetate was observed at 500 mg/ml against S. pneumonia with a diameter of 17.5 mm while the lowest zone of inhibition at the same concentration was recorded against Klebsiella sp with a diameter of 11.5 mm.
Chloroform extract: In a similar manner as Ethanol and Ethyl acetate extracts, the chloroform extract exhibited antibacterial and antifungal activities against the test isolates with zones of inhibition against bacteria isolates ranging from 7.50 mm at C1 - 17.5 mm at C5. It was also comparatively better in antimicrobial performance than the control. The extract exhibited inhibition against tested fungal isolates in the range of 9.0 mm at C2 - 17.5 mm at C5. The highest zone of inhibition was also recorded at 500 mg/ml against B. cereus and A. niger with a diameter of 17.5 mm in each case, while the lowest zone of inhibition for the same concentration was recorded against Klebsiella sp with a diameter of 10.0 mm.
Comparative activities of extracts: Ethanolic extract performs better than ethyl acetate and chloroform extracts as it recorded the highest zone of inhibition of 20.5 mm against Streptococcus pneumoniae while ethyl acetate and chloroform recorded 17.50 mm each against Streptococcus pneumoniae and Bacillus cereus, respectively (Table 4). The extracts of Terminalia glaucescens seem to exhibit a good antibacterial activity against Streptococcus pneumoniae and Bacillus sp and lesser activity against Klebsiella sp. The extract exhibited higher antifungal activity against Trichoderma sp. and Aspergillus niger (Table 4).
Crude extract of ethanol at the highest concentration C5 tested was a more potent antimicrobial agent when further compared with standard (Gentamicin, Streptomycin and Nystatin). Ethanolic extract recorded higher zones of inhibition against Pseudomonas aeruginosa, Klebsiella sp and E. coli as 14.5 mm, 13.5 mm and 20. 0 mm at C5 while Streptomycin recorded 11.5 mm, 10.5 mm and 10.0 mm, respectively, against the same set of organisms (Table 5). The extract recorded highest zones of inhibition against Streptococcus pnuemoniae and Bacillus cereus as 20.5 mm and 19.0 mm at C5 while the record of Gentamycin against the organisms were 13.5 and 13.0 mm, respectively. The ethanolic extract equally exerted zones of inhibition that were comparatively higher than that of nystatin against the tested fungi, but were not statistically different from the values obtained for nystatin (P<0.05). Higher activities were recorded against Aspergillus flavus, Aspergillus niger and Trichoderma sp; 19.0 mm at concentration C5, while the least was recorded as 15.0 mm against Candida sp at the same concentration. The antimicrobial activity of ethanol was therefore notable against all tested organisms and commercial antibiotics at C5 (P<0.05). The results are presented in Table 5. Plates (1 and 2) illustrates reported characteristic antibacterial (against E.coli) and antifungal (against A. flavus) activities of ethanolic extracts.

C – Control consisting of solvent without crude extract,
25-500 – Amount of crude extract dissolve in 1 ml of solvent.
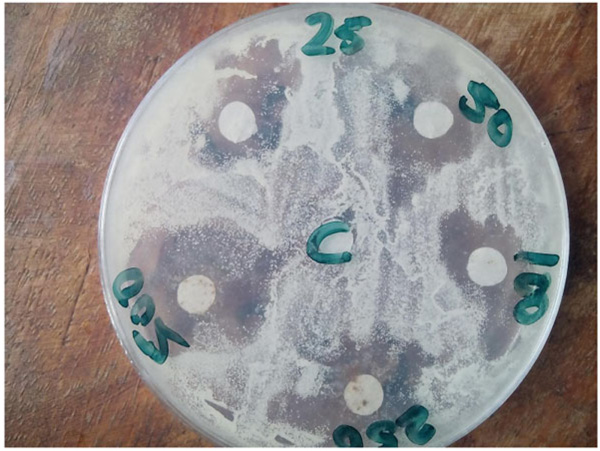
C – Control consisting of solvent without crude extract
25-500 – Amount of crude extract dissolve in 1 ml of solvent.
3.2. Phytochemical Screening of Terminalia glaucescens
The results obtained from the qualitative phytochemical screening of Terminalia glaucescens are presented in Table 6. In ethanol, saponins, tannins, alkaloids, flavonoids, terpenoids, steroids and anthraquinones were present while reducing sugars and phlobatannins were absent. In ethyl acetate and chloroform, saponins, tannins, alkaloids, terpenoids, steroids and anthraquinones were present while Reducing sugars, Phlobatannins and Flavonoids were absent.
| Ethanol | Ethyl Acetate | Chloroform | |
|---|---|---|---|
| Saponins | + | + | + |
| Tannins | + | + | + |
| Reducing Sugars | - | - | - |
| Alkaloids | + | + | + |
| Flavonoids | + | - | - |
| Terpenoids | + | + | + |
| Phlobatannins | - | - | - |
| Steroids | + | + | + |
| Anthraquinones | + | + | + |
- = Absent
4. DISCUSSION
4.1. Comparative Activities of Plant Extracts and Commercial Antibiotics
Tables 2 to 5 present the summary of the antibacterial and antifungal activities of the various extracts of Terminalia glaucescens and that of selected commercial antibiotics against different fungal and bacterial strains tested. Antimicrobial activities were found in all the plant extracts and with remarkable zones of inhibitions. The antibacterial and antifungal activities of active extracts were observed in the increasing order of Ethanol>Chloroform≥ethyl acetate with respect to the maximum zone of inhibition. Ethanolic extract compared favourably against commercial streptomycin, gentamycin and nystatin which were used as standards with significant statistical differences. The zones of inhibition reported in this study against bacterial isolates tested ranged from 7.5 to 20.5 mm while that recorded against fungal isolates ranged from 8.0 to 19.0 mm. The extent of antimicrobial activity of extract based on the zone of inhibition has been described as low (12-18 mm), moderate (19-22 mm) and strong (23-38 mm) [47], we may therefore infer that ethanolic extract of T. glaucescens which exhibited inhibitions in a range of 18.0-20.0 mm against most of the microbial strain tested has considerable antimicrobial activity. Previous screening by earlier researchers had demonstrated the antimicrobial efficacy of variuos plant extracts including Holarrhenea antidyssentrica [48]; Tapinthus senssilifolius [49]; Rauelfia tetraphylla and Physalis minima [50]; Achillea santolina, Salvia dominica and Salvia officinalis [51]; Psidium guajava and Mangifera indica [52] and Salicornia brachiata [53] against different bacteria and fungal isolates. Viji et al. [17], have demonstrated that ethanolic and chloroform extracts can potentially be used against E. coli, Pseudomonas aeruginosa, Bacillus sp and Klebsiella sp. which were used in this study.
4.2. Phytochemicals
Plants are known to contain a number of phytochemicals such as flavonoids, saponins, tannins and other phenolic compounds that have antimicrobial activities [54-56]. The results of phytochemical screening in T. glaucescens reveal the presence of saponins, tannins, alkaloids, terpenoids, steroids and anthraquinones in all extracts investigated. Reducing sugars and phlobatannins were absent. Flavonoids was however present in ethanolic extract but not in ethyl acetate and chloroform extracts. The presence of antifungal and antimicrobial substances in higher plants is well established as they have provided a source of inspiration for novel drug compounds [17]. This suggests that the antimicrobial activities of the plant under investigation may be as a result of the phytochemicals present.
4.3. Antimicrobial Responses and Mechanisms
It was noted that all the extracts exhibited antibiosis against gram positive and gram negative bacteria as well as the tested fungal isolates (broad spectrum activities). Noteworthy, is the activity of the ethanolic extract at 500 mg/ml against Streptococcus pneumoniae (20.5 mm), E.coli (20 mm) and B. cereus (19.0 mm). The values of zones of inhibitions show that ethanolic extract performed better than ethyl acetate and chloroform extracts. The susceptibility of the tested microbes to the extracts varies across the different solvents of extraction. This suggests that ethanolic extracts of T. glaucescens was more effective than ethyl acetate and chloroform extract. The variation in responses of the various microbial strains to individual extracts may be attributed to the nature of the microbial cells and their genetic diversity [57]. The chemistry of the extracting solvent and subsequent bio-active components extracted by various solvents may also explain the difference in antimicrobial potency of the extract in different solvents [16], which may affect overall activity [57]. Resistance could be due to the permeability barrier provided by the cell wall or to the membrane accumulation mechanism [58].
Variation in phytochemical components of the various extracts may result from variance in the chemistry of the extracting solvent which selectively affect extraction of various bio-active metabolites. The mechanisms of antimicrobial actions of these compounds may be via cell membranes perturbations [59] and may involve diverse molecular modes, such as binding and increasing the permeability of cell wall and membrane component [60]. These induce membrane destabilization, leakage of cytoplasmic contents, loss of membrane potential, change of membrane permeability, lipid distribution, the entry of the peptide and blocking of anionic cell components or the triggering of autolytic enzymes and the final death of the microbial cell [61]. This finding is similar to that of Dahot [62] who reported that plant extract from M. oleifera had antimicrobial activity against E. coli, S. aureus and B. subtilis. Dahot [62], however, reported resistance from Aspergillus niger and Aspergillus flavus from which notable susceptibility was recorded in this study. The curative advantage of such a plant used in this finding is that consumers including animals tend to consume the plant material in large quantities and in high concentrations. This suggests the ability of the plant to meet the required physiological levels to inhibit the pathogenic growth in situ and point to the potential of T. glaucescens as biocides to be explored in various fields. The differences in ethanolic extract activity from that of ethyl acetate and chloroform extracts as well as the similarities in the activities of ethyl acetate and chloroform extracts follow the pattern observed in the similarities and differences in phytochemical compositions of the extracts (Table 6). This may suggest the positive contributions of these phytochemicals in the antimicrobial activities of the extracts.
There exist reports to show that the mechanism of water purification by plant material is associated with their flocculating, coagulative and disinfecting properties [63]. Polysaccharides as well as protein associated phyto-chemicals have been implicated in the purification process. The phytochemicals may form flocs which settles slowly while sweeping out suspended impurities in water. Phytochemicals with their net charge; either positive, negative or neutral are thought to combine with active sides of colloids and impurities; such interaction produces a bridging effect, binding impurities and phytochemicals together into a large particle which settles under the action of gravity. The disinfecting properties of lemon and moringa extracts in water have been partly associated with ability to alter the pH of water, making it unsuitable for some living contaminants [5,64,65] while the effectiveness in coagulation and colour reduction is a function of particles size and concentration [66].
While some of the current chemical compounds like alum and chlorine used in disinfecting water has been tagged a precursor for cancer, as it forms tetrahalomethane compounds and lead to hormone mimics as well as generating dementia in young and elderly, reports exist to show that plant based technology are at different stages of development for water purification. The technology will be very simple, non-toxic and with no major machinery nor specialized labour needed [5,67].
In this study, T. glaucescens extracts had a dose dependent bactericidal properties against all bacterial strains which are mostly known to be multi-resistant [6, 8]. According to several authors, these bacteria are generally less sensitive to the activity of plant extracts [68, 69]. The ability of the extracts to antagonise and exhibit broad spectrum antibiosis is therefore noteworthy. The plant ethanolic extracts with optimal antimicrobial performance can therefore be recommended for further research in development of potent antimicrobial agents against multidrug and emerging resistant microbes.
CONCLUSION
The present study which was aimed at establishing the antimicrobial efficacy of different crude extracts of T. glaucescens showed that the crude extracts from the plant possessed potent activity against the employed bacteria and fungi. Similarly, phytochemical screening showed that the antimicrobial activities of the crude extracts of the plant may depend on the presence of phytochemicals such as saponins, tannins, alkaloids, flavonoids, terpenoids, steroids and anthraquinones. This plant crude extracts could serve as potential sources of new antimicrobials and for green and eco-friendly water treatment technology development.
RECOMMENDATIONS
Further research is needed towards isolation and identification of active metabolites present in the extracts which could be adopted for different biotechnological uses.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared None.


