All published articles of this journal are available on ScienceDirect.
Mutagenic Potentials of Potable Water From Ground Sources
Abstract
Background:
The presence of compounds with mutagenic activity in drinking water by means of short-term mutagenicity tests have been revealed in many studies. The influence of the different water treatment steps on the mutagenicity of some drinking water samples were evaluated using the Ames test.
Method:
Four different types of samples were collected from four water treatment factories within Port Harcourt metropolis: raw water from borehole (1), water after sand and granular activated carbon filtration (2), water after reverse osmosis (3), and water after Ozone and UV treatment (4). These samples were subjected to mutagenicity test using two mutant strains of Salmonella typhimurium (TA 100 and TA 98) without S9 activation enzyme.
Result:
The mutagenic analysis results revealed that raw water samples from Kent and Rivoli table water products showed mutagenic potential with TA100 and TA 98, respectively. But Kent table water showed more mutagenic potential than Rivoli and Fressi table water samples. Fressi table water is predominantly cytotoxic with all the treatment processes except for UV treatment with TA 98 strain. The finished products (water after ozone and UV treatment) of Kent table water and Rivoli table water also showed mutagenic potentials higher than those treated with TA100 and TA98 without S9 mix, respectively. Only the samples treated with activated carbon showed highly reduced mutagenic potential.
Conclusion:
This study highlights the mutagenic effects of water treatment as another quality assessment option for assessing the portability of water samples. Water treatment with activated carbon can be reintroduced after disinfection with ozone/ultraviolet to eliminate possible mutagenic by-product in the finished product.
1. INTRODUCTION
Good quality drinking water may be consumed without adverse effect on health. Such water is said to be “potable” when it is free from inorganic and organic substances, is aesthetically acceptable, free of objectionable taste, color, turbidity, and odor [1, 2]. One of the major sources of potable water supply throughout Nigeria is ground water. However, many chemical contaminants have been identified in ground water mainly from industrial and agricultural practices [3-5]. These chemicals can have mutagenic, genotoxic and carcinogenic effects [6, 7]. The potential presence of genotoxins in water results not only from anthropogenic activities such as pharmaceutical, biocidal and industrial chemical contamination, but also from other various water treatment methods [8, 9]. Disinfection of drinking water to remove and inactivate pathogens by chlorination, ozone and UV-irradiation has been shown to release by-products that can be potentially genotoxic on testing with short-term mutagenicity tests [9]. Mutagenic compounds could also be derived from corrosion or leaching from the internal surface of the water tanks and pipelines [10] which are frequently coated with coal tar or other materials such as plastics. Formation of mutagens during water distribution, as a result of chemical reaction (e.g. reaction of residual chlorine with natural organic matter during water treatment) or microbiological action, may also be possible [11]. It is therefore needful that the quality of groundwater is protected and public health not compromised by carrying out proper treatment of ground water prior to its distribution. Several reports have indicated that only a small percentage of drinking water is obtained from ground water. For example, Norway takes only 13 per cent of its drinking water from groundwater sources, whereas Austria and Denmark use groundwater resources almost exclusively for drinking water supply [12]. However, throughout Nigeria, individuals, communities, local, state and federal governments have been sinking wells and boreholes to tap the rich ground water resources for human use and irrigation purposes [13]. Serious health challenge can result from certain treatment processes carried out by drinking water factories which may be toxic and introduce mutagens. Thus, the need to evaluate the mutagenic potentials of water treatment processes of some water factories in Rivers State became necessary.
2. MATERIALS AND METHODS
2.1. Research Design
Three water factories were randomly selected for this study; Kent table water, Rivoli table water (Choba Road) and Fressi table water (Rumuokwuta), all in Rivers State). With the consent of the factory managers and quality control managers, bottle water samples were drawn.
2.2. Samples and Sampling Technique
Bottled water samples were collected from the above water treatment plants according to each factory’s treatment processes. The steps of drinking water treatment processes took place in the following sequence: 1) sand and granular activated carbon (GAC) filtration; 2) reverse osmosis; (3) ozone disinfection; and (4) UV disinfection (finished product). The bottle water samples collected comprised raw water from borehole, water after pre-treatment i.e. sand and granular activated carbon filtration and finished product i.e. water after reverse osmosis, disinfection with ozone and ultraviolet (UV) light. Samples were serially collected in sterile 500 mL polyethylene terephthalate (PET) bottles after each water treatment step for mutagenic analyses. The samples were then transported to the microbiology laboratory of the University of Port Harcourt, in an iced packed cooler for immediate mutagenic analyses.
2.3. Ames Fluctuation Test
Ames fluctuation test was used for mutagenic analysis using the Muta- Chromo Plate Ames test kit [14]. The test strains Salmonella typhimurium (lyophilized) TA98 and TA 100 were used without S9. The test strains were rehydrated overnight prior to the assay by incubation at 370C for 16-24h and subsequently examined for cell multiplication.
2.4. Mutagenic Analysis
Drinking water samples of about 100 mL each (raw water, water after sand and carbon filtration, water after reverse osmosis, water after ozone disinfection and water after UV disinfection) were filter-sterilized using 0.22 μm sterile filter supplied with the kit in triplicates. Filter-sterilized samples of about 17.5 mL was aseptically dispensed into each sterile tube and labeled appropriately. The reaction mixture was aseptically prepared by measuring and mixing 43.24 mL from bottles (A) + 9.5 mL from bottles (B) +4.76 mL from bottles (C) + 2.38 mL from bottles (D) + 0.12 mL from bottles (E) into the reaction mixture container supplied with the kit. About 2.5 mL volume of the above reaction mixture was aseptically dispensed into each sterile tube containing 17.5 mL of the sample and mixed thoroughly to a final volume of 20 mL. A blank containing 17.5 mL of distilled water and 2.5 mL of reaction mixture was prepared. Bacterial suspension from the overnight culture of about 5 μl volume was pipetted into each sample tube (with the exception of the reaction blank tube) and mixed thoroughly. Negative control used was a background plate containing 17.5 mL of distilled water, 2.5 mL of reaction mixture and 5 μl of bacterial culture. The positive controls for the two strains TA100 and TA98 were conducted using the standard mutagens sodium azide and 2-nitrofluorene respectively. Sodium azide of about 0.1mL (NaN3, 0.5 μg/100 μl) and 2-nitrofluorene (2-NF 30 μg/100 μl) were pipetted into sterile tube containing 17.4 mL of distilled water. The content of each tube (now containing test sample, reaction mixture and bacteria) was poured into the multichannel pipette reagent boat. Using the eight (8) channel multipipette, 200 μl aliquots of mixture was dispensed into each well of a 96-well microplate (200 μl per well). The plates were covered with a lid, sealed in sterile airtight Ziploc bags to avoid evaporation and incubated at 37oC for 5 days. After the incubation period, the plates were removed from the incubator and scored for mutagenic activity.
2.5. Analysis of Results
The mutagenic activity of the incubated blank plates was investigated for sterility check by scoring the plates using the Muta-Chromo Plate Ames test kit instructions (EBPI Inc., Mississauga, Ontario, Canada) [14]. The blank sterility check was observed, and the assay is considered contaminated if any changes occurred in the plate. The result of each treatment plate is scored against the background mutation. The number of wells that scored positive in the treatment plate and the number of wells that scored positive in the background plates were recorded. The background plate shows the level of spontaneous or background mutation of the assay organism. When the blank plate indicates a purple it signifies that the assay was aseptically carried out; all yellow, partially yellow or turbid wells were scored as positive and all purple wells were scored as negative.
2.6. Data Analysis
The number of positive (yellow) wells out of 96 wells per plate was compared with the number of spontaneous revertant wells obtained with the background. The results are expressed as a mutagenicity ratio (MR): MR= number of positive wells in samples/number of positive wells in the negative control [9]. Mutagenic ratio, (MR) > 2.0 shows mutagenic risk [15-18]. A sample was considered mutagenic when a statistically significant increase occurred in the number of positive wells compared with spontaneous revertant wells in the background plate [9]. Statistical significance was determined using the Chi-square (x2) analysis illustrated by [19].
3. RESULTS
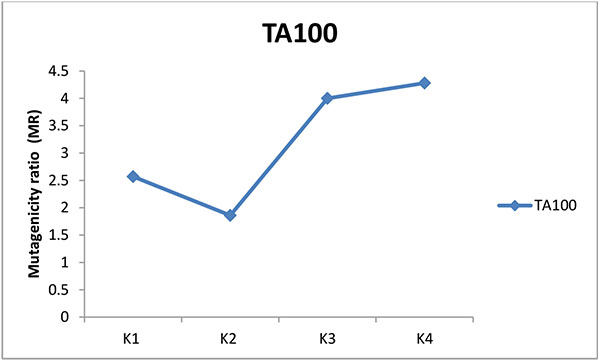
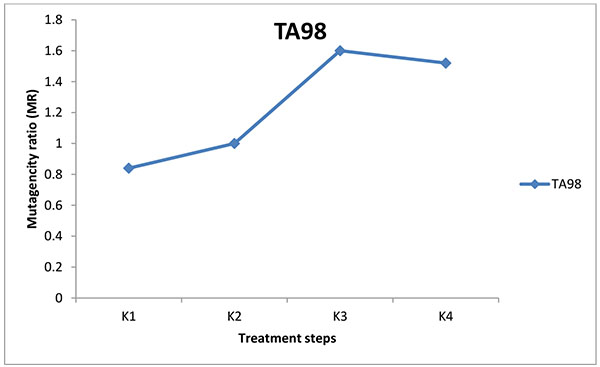
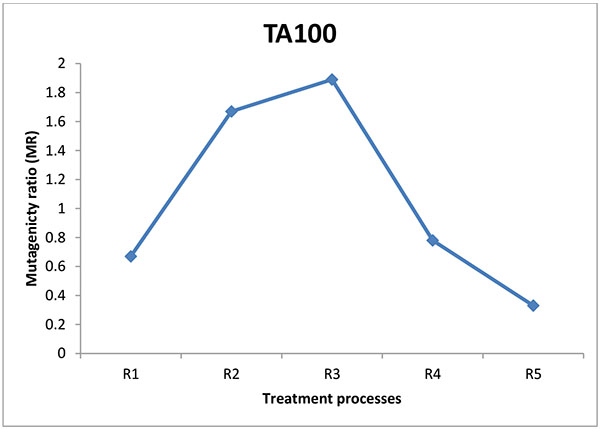
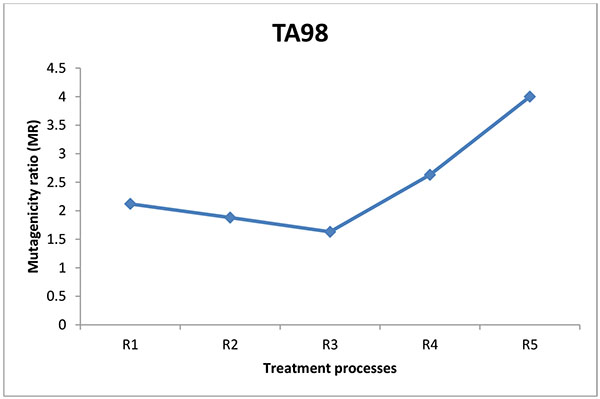
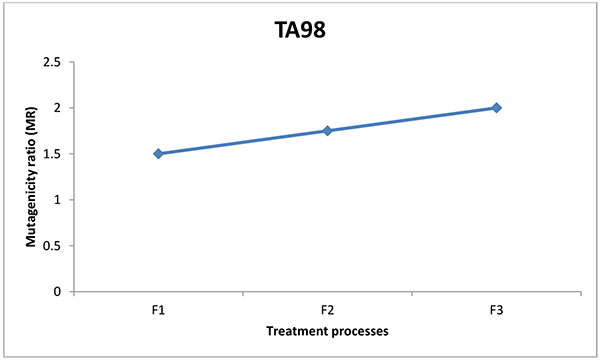
The mutagenic potential of the water samples from three water factories in Port Harcourt were analysed using Salmonella typhimurium strains TA100 and TA 98 without S9 mix (Figs. 1-6). The results obtained from each water factory shows mutagenicity ratio (MR) of each treatment step with MR > 2 as being mutagenic. Figs. (1) and (2) show results from Kent table water treated with strains TA 100 and TA 98, respectively. They showed various responses of the strains to the water after each stage of water treatment. MR was higher than 2 for raw water treated with TA 100 showing significant mutagenesis but lower for treatment with TA 98. The four (4) samples from Kent showed mutagenic potential with strain TA 100. Figs. (3) and (4) show results obtained from Rivoli table water. The raw water sample, water treated with ozone and water after UV light had mutagenic ratios higher than 2 upon treatment with strain TA 98. The mutagenic ratio was lower than 2 across all the samples treated with strain TA 100 indicating insignificant mutagenesis. Figs. (5) and (6) show results of samples from Fressi table water upon treatment with strains TA 100 and TA 98, respectively. These results showed predominant cytotoxicity except for the UV water sample treated with TA 98. This implies that the mutation rates observed in the cytotoxic samples were below the natural spontaneous mutation rate of the strains with slightly significant mutagenesis.

4. DISCUSSION
The mutagenic activities of water samples from three bottle water factories were analyzed using Ames mutagenicity test. From the above results, raw water (K1) from Kent table water showed mutagenic potential with TA100. These unidentified substances might be natural organic substances found in the ground source. Water after sand and carbon filtration (K2) showed cytotoxic response. Water after reverse osmosis (K3) and water after ozone/Ultraviolet rays treatment (K4) showed significant mutagenicity with TA100 without S9 activation but not with TA98. For Rivoli table water, mutagenic risk was observed in raw water with TA98, water after ozone treatment (R4) and finished product treated with ultraviolet radiation (R5). The samples did not show mutagenic risk with TA100, implies that frame-shift mutagens are responsible for this mutagenic risk [20]. For Fressi table water, only the finished product treated with Ozone and Ultraviolet rays showed mutagenic risk but other samples showed cytotoxicity [21]. From this study, the finished products had mutagenic potential with Kent table water showing the greatest mutagenic risk which might be from natural products in the raw water or from run-offs such as industrial/agricultural contamination of the source water and products arising from drinking water treatment and/or distribution [22-24]. Disinfectants such as chlorine can introduce non-volatile mutagens in drinking water [21]. The mutagenic activity appears to originate primarily from the reactions of chlorine with the humic substances in water sources [24]. In some locations, mutagenic contaminants from agricultural or industrial sources contribute enormously to the mutagenicity of the finished potable water [21]. However, the factories understudied in this research did not admit to using chlorine for disinfection. Although, the trihalomethanes in chlorine-treated and non-volatile mutagens in drinking water were not determined, it is expected that the water contain some of these mutagenic substances. According to Kent table water, their raw water is treated with ozone before passage into the treatment plant. Park et al. noted that production of mutagens is greatly dependent on chlorination pH with a pattern of decreasing mutagenic activity with increasing pH [21]. The criterion for mutagenic potential used in this study revealed raw water from Kent showing the highest mutagenic risk with TA100 followed by Rivoli raw water with TA98 without S9 mix. Apart from the finished product of Fressi table water, other samples showed cytotoxicity. Similar tendencies occurred with the reports of [25-27]. From these conventional water-purifying processes, mutagenic activities are high in raw water, decreased through sand and carbon filtration and increased after post-treatment with ozone/Ultraviolet light. This work has not been replicated lately in Nigeria but the results are in line with already published works determining mutagenicity of other water treatment processes.
CONCLUSION
Mutagenicity tests of water treatment processes of potable water from ground sources using Ames test demonstrate that these water sources contain many unidentified and unregulated toxicants which are further changed by the treatment processes. The raw water sample and finished product (K1 and K4) of Kent water showed significant mutagenic potential with TA100 and not with TA98. This implies that Kent source water contain point mutagens. For Rivoli water, the raw water and finished products (R1 and R5) were mutagenic with TA98 and not with TA100. Water after treatment with reverse osmosis showed very slight mutagenicity. Fressi table water was mainly cytotoxic showing that the number of positive wells were mostly below the strain’s natural spontaneous rate, indicating that several unidentified substances might be responsible for the cytotoxicity. Kent table water samples showed the most mutagenicity. With the exception of Kent table water, mutagenic activity was reduced after carbon filtration for both TA100 and TA98 of other table water samples analyzed.
RECOMMENDATIONS
- Activated carbon known to remove mutagenic substances during the water treatment can be re-introduced after disinfection with ozone and UV. This will ensure that any mutagen formed from disinfection with ozone and UV will be removed before the water gets to the final consumer.
- Regulatory bodies should enlighten bottle water factories on the continual need to give adequate treatment to the potable water from ground sources.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared None.


