All published articles of this journal are available on ScienceDirect.
Phytodecontamination of Water Systems from Phenolic Endocrine Disruptors and the Regulation Role of Natural Organic Matter
Abstract
In the last decades an increasing number of natural and synthetic compounds have been recognized as endocrine disruptors (EDs) because of their hormone-like activity and capacity to alter the normal hormonal functions of animals and humans. Among EDs, there are phenolic compounds widely present in terrestrial and aquatic systems, such as bisphenol A, 4-nonylphenol (NP), 4-tert-octylphenol, estrone, ethynilestradiol and so on. Estrogenic effects of these molecules have been ascertained on mollusks, crustaceans, fishes, amphibians and mammals starting from concentrations of 1 μgL−1. Thus, the removal of EDs from polluted media is a priority goal in order to avoid risks for the ecosystem health. Nowadays, several physico-chemical methods are mainly used for the removal of EDs from liquid and solid matrices. Nevertheless, these methods are expensive, difficult to apply and may produce a negative impact on the environment. Recently, most of studies on soil and water remediation from EDs address more sustainable techniques using bacteria, fungi, microbial enzymes and plants. Phytoremediation uses photoautotrophic organisms to uptake, transform, volatilize or stabilize pollutants present in waters, sediments, soils and atmosphere. As this technology is solar driven and exploits natural sources, it is consequently environmentally safe and cost-effective. A fundamental role in the phytoremediation process is played by natural organic molecules, mainly dissolved organic matter and humic substances. These compounds are ubiquitous in all terrestrial and aquatic environments and they interact at various extent with all contaminants deriving from agricultural, industrial and urban activities. Natural organic matter has a relevant biological activity and may also regulate the decontamination capacity of plants and other organisms, such as algae and fungi. In this review, some results of phytodecontamination studies conducted using herbaceous plant species which are presented and discussed. Further, the modulation role of natural organic matter on the phytodecontamination process is highlighted.
PHYTOREMEDIATION
In the last decades, the increasing human activities have contributed markedly to the environmental pollution, particularly regarding natural waters. The huge number of organic and inorganic contaminants present in natural systems impose the need to intervene with specific technologies to restore the original status of these sites accomplishing environmental standards set by laws. Series of national legislative decrees along with European directives regulate the remediation and environmental restoration of contaminated sites, defining the procedures and criteria for the elimination of pollution sources and the reduction of concentrations of pollutants according to the principles and rules of the European Community.
Among the numerous methodologies explored and tested in recent years for remediation, phytoremediation has conquered increasing attractiveness - as a viable alternative towards traditional engineering-type techniques, mainly physico-chemical - owing to its efficiency and sustainability in terms of eco-compatibility and low costs [1]. In fact, both in situ and ex situ phytoremediation techniques use plant organisms with reduced needs, easy to grow, monitor and adaptable to different edaphic and climatic conditions. Further, plants give a valuable contribution to soil fertility both directly, through root expansion and exudation, and indirectly promoting the growth and activity of microorganisms. Finally, an added-value of phytoremediation originates from the aesthetic value of plants. All these aspects make this technology very competitive and attractive with a broad consensus by public opinion.
Despite the great potential of phytoremediation, in open field applications the following aspects may limit its success: (i) the confinement of the roots to the surface layer of the contaminated matrix; (ii) the longer time needed in comparison with conventional decontamination techniques; (iii) the susceptibility of plants to abiotic stresses and biotic attacks; (iv) the possible low tolerance of plants towards the pollutant concentration; (v) the possibility to remove only hydrophilic and moderately hydrophobic compounds.
The various phytotechnologies developed are based on the basic physiological processes occurring in higher plants and associated microorganisms, such as transpiration, photosynthesis, metabolism and mineral nutrition [2]. Phytoextraction (also known as phytoaccumulation, phytoabsorption, or phytosequestration) consists in absorbing pollutants from soil through the roots and then translocate them to the stem or leaves. After an adequate period, the removal of aerial plant organs ensure the permanent decontamination of the site. That is particularly important for the removal of metals. Rhizofiltration is another process that plants adopt to uptake metals in aquatic environments. Several aquatic species are very effective in removing heavy metals from water and wastewaters [3]. However, some of these plants, such as pennywort (Hydrocotyle umbellate L.) [4] and duckweed (Lemna minor L.) [5], have small and slow-growing roots that represent a limit to their use for remediation. Terrestrial plants, such as sunflower (Helianthus annuus L.) and Indian mustard (Brassica juncea Czern.) [6], are more suitable for rhizofiltration, since they produce longer, thicker, fibrous root systems with large surface areas for the sorption of pollutants. Phytostabilization (or phytoimmobilization) relies on the ability of plants to reduce the mobility or bioavailability of pollutants and thereby avoiding their dispersion by leaching or erosion phenomena and possible entrance into groundwater or food chain. However, this mechanism is only a temporary solution, because the pollutants remain in the contaminated matrix. Plants can immobilize heavy metals in soils by means of sorption by roots, precipitation, complexation or valence reduction in the rhizosphere [7-9]. Phytostabilization results more efficient in fine-textured soils with high organic matter content. Phytopumping is a technique used for both organic and inorganic pollutants that uses plants as organic “pumps” to uptake large volumes of contaminated water as part of the transpiration process [10]. The plants that are capable to remove large amounts of water from the soil, such as willow (Salix spp.), are most suitable for this technique. Gatliff [11] has shown that Salix spp. are capable of taking 200 liters of water per day, thus being able to decontaminate large amounts of groundwater in shallow aquifers. A relevant role for organic pollutants has played by phytodegradation or phytotransformation. This process regards the capacity of some plants to degrade or transform organic contaminants by means of their enzymes. Xenobiotics absorbed by plants can follow different pathways: (i) absorption and relocation in tissues; (ii) complete degradation; and (iii) partial degradation and volatilization. Some plants, named as “Green Liver” for the biosphere, are able to accumulate and detoxify organic xenobiotics collected from contaminated sites through their metabolism. Degradation of phenols was achieved by horseradish, potato and white radish [12, 13], and trichloroethylene in soil and groundwater was efficiently transformed by poplar trees [14, 15]. Another plant process is phytovolatilization and occurs when, under particular conditions, some contaminants absorbed by plants are converted into volatile forms and transferred into the atmosphere through the transpiration process. As a consequence of a partial degradation, some organic xenobiotics, such as trichlorethylene, may become volatile and be transpired by plants through the leaves and stem. However, this phenomenon could generate volatile catabolites of greater toxicity, which would invalidate the decontamination efforts and relocate the contaminant in soil. Rhizodegradation concerns an indirect action of plants which produce root exudates – containing carbohydrates, organic acids, amino acids, flavonoids etc. - that stimulate the degradative metabolism of microorganisms in the rhizosphere. In addition, plants themselves can release enzymes capable of degrading the organic contaminants in the soil. Hedge and Fletcher [16] have shown that phenolic compounds released by red mulberry (Morus rubra L.) roots may stimulate the degradation of recalcitrant pollutants by microorganisms. Finally, phytodesalination is an emerging technique that uses halophytes to remediate salt-affected soils restoring the normal plant growth [17, 18].
The choice of the proper phytoremediation technique in vivo and in vitro applications can guarantee the success of the depollution process. A technique of in vivo phytoremediation could be adopted where the contaminant is not in contact with the plant, like in the case of deep aquifers. In such case, the contaminant may be extracted with various technologies and then transferred in suitable areas for the phytoremediation. After treatment, the water or the soil decontaminated can return to their original place, while plants can be harvested and the biomass recycled. As expected, this technology is quite expensive. In a process of in vitro phytoremediation, plant extracts, such as enzymes, are used in decontaminating polluted matrices. Apparently, this technology is the most expensive, because of the high costs of extraction and preparation of enzymes, but some plants, such as Artemisia dracunculas var sativa, are very efficient in secreting enzymes under stressed conditions, thus lowering the cost of the treatment [10]. Unfortunately, these enzymes are easily degradable in soil conditions that might be a limit of this application. An in vitro technique has the advantage of applicability in different environmental conditions and for sites not accessible to plants.
Numerous plant species have shown an appreciable phytoremediative ability towards different classes of organic contaminants. However, a number of these plants cannot be safely used because of their tendency to be invasive or potentially invasive. Some plant species used in phytoremediation from phenolic endocrine disruptors are referred in Table 1.
ENDOCRINE DISRUPTORS
Endocrine disruptor compounds (EDs) are a wide group of persistent organic pollutants known or suspected to interfere either directly or indirectly with the normal functioning of the endocrine system of animals and humans by acting as hormone-like substances [19-21]. Exposure to EDs may severely compromise human reproductive functions and success [22], and can lead, especially during the stages of pregnancy and lactation, to serious and long-lasting disturbances of endocrine functions [23]. EDs may enter fresh and sea water due to agricultural practices and by application, discharge and disposal of urban and industrial effluents, sludges, and other wastes. The environmental fate of some endocrine disruptors has been extensively investigated [21, 24-26].
Some plant species used in phytoremediation from phenolic endocrine disruptors.
| Plant Species | Compound | Reference |
|---|---|---|
| Chlorella fusca | Bisphenol A | [58] |
|
Cyndodon dactylon, Festuca arundinacea, Lolium perenne, Agropyron fragile, Trifolium repens, Cucumis sativus, Cucurbita pepo, Raphanus sativus |
Bisphenol A | [39-41] |
| Dracaena sanderiana, D. fragrans | Bisphenol A | [61] |
|
Eucalyptus perriniana Glycine max, Triticum aestivum |
Bisphenol A | [62] |
| Digitalis purpurea, Datura stramonium | Bisphenol A | [42] |
| Monoraphidium braunii | Bisphenol A | [56] |
| Phragmites australis | Bisphenol A | [63] |
|
Portulaca oleracea Pseudokirchneriella subcapitata, Scenedesmus acutus, S. quadricauda |
Bisphenol A | [53] |
| Coelastrum reticulatum | Bisphenol A | [60] |
| Salvia spp. | Bisphenol A | [64] |
| Stephanodiscus hantzschii | Bisphenol A | [57] |
| Vicia faba, Lycopersicon esculentum, Triticum durum, Lactuca sativa | ||
| Bisphenol A | [43] | |
| Raphanus sativus, Lolium perenne | 17α ethynilestradiol | [40] |
| Portulaca oleracea | 17β estradiol | [53] |
| Portulaca oleracea | octylphenol | [53] |
| Crested wheatgrass | 4-Nonylphenol | [44] |
| Lolium perenne, Brassica rapa | 4-Nonylphenol | [45] |
| Lupinus spp. | 4-Nonylphenol | [49] |
| Portulaca oleracea | 4-Nonylphenol | [53] |
| Raphanus sativus, Lolium perenne | 4-Nonylphenol | [39] |
Among EDs there are a number of phenolic compounds commonly used in industrial processes and anthropogenic activities, such as bisphenol A, nonylphenol, octylphenol, estrone, estradiol, ethinilestradiol and so on. Bisphenol A (BPA) is used as an intermediate compound in the preparation of epoxy resins and polycarbonates, and as a stabilizer for plastics such as polyvinyl chloride. BPA is present in a wide range of manufactured goods, such as adhesives, food and drink packaging, electrical and electronic parts [24, 27]. 4-nonylphenol (NP) is a component of nonylphenol ethoxylates which are a group of surfactants used as emulsifiers for pesticides, antioxidants for rubbers and plastics, additives for lubricant oils, and in many other applications [28]. The 17α-ethynilestradiol (EE2) is a synthetic steroid hormone used as component of contraceptive pills. A number of works have been conducted to remove these compounds from natural and anthropogenic water systems using plants. In this review we discuss results of phytoremediation from BPA, NP and EE2 during the germination and growth of a number of herbaceous plant species. Some mechanisms involved in the phytoremediation process are also considered.
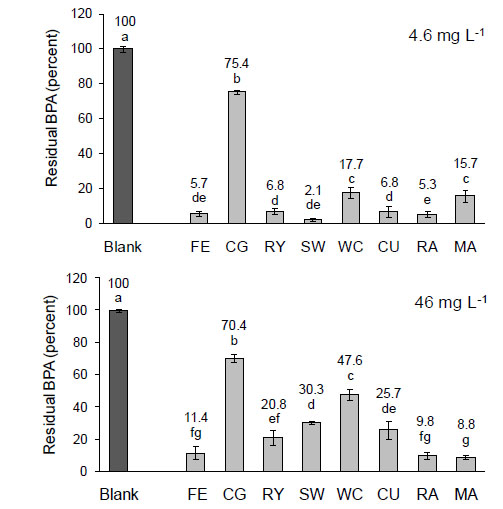
Residual BPA measured in the blank (without seeds) and in the aqueous medium at the end of germination in the presence of BPA at two concentrations for fescue (FE), couch grass (CG), perennial ryegrass (RY), Siberian wheatgrass (SW), white clover (WC), cucumber (CU), radish (RA), and marrow plant (MA). Values with the same letter are not significantly different according to the least significant differences (LSD) test at probability ≤ 0.05. The vertical line on each bar indicates the standard error for five replicates. [From Loffredo et al. [41] with permission].
THE INFLUENCE OF NATURAL ORGANIC MATTER ON PHYTOREMEDIATION
The distribution and availability of EDs in soil and water systems depend on several factors, including the physical and chemical properties of the compounds and the type and extent of their interaction with the inorganic, organic and biological components of the environmental system. Generally, the affinity of EDs for organic colloids is greater than that for mineral colloids. Thus, the content and nature of suspended and dissolved organic matter, and especially its humified fractions, play a major role in determining the fate of EDs in the natural system.
Approximately 40-60 % of the total organic carbon occurring in natural waters is represented by humic substances (HS) that are ubiquitous natural non-living organic materials. HS consist of relatively high-molecular-mass, yellow-to-black colored organic compounds of mixed aliphatic and aromatic nature, formed by secondary synthesis reactions (humification) of products of microbial and chemical decay and transformation with recalcitrant residues of biomolecules originated from organisms during life and after death [29]. On the basis of their solubility in water at different pH, HS are divided into two main fractions, humic acids (HAs), which are soluble in dilute alkaline solution, and fulvic acids (FAs), which are soluble at any pH values e.g., [29]. HAs and FAs cannot be regarded as single chemical entities described by unique, chemically defined molecular formulas, but they can be operationally described by model structures constructed on the basis of available chemical and physicochemical data e.g., [29, 30]. A “typical” model macromolecule of soil HA basically consists of aromatic, phenolic, quinonic and heterocyclic “building blocks” that are randomly condensed or linked by aliphatic, oxygen, nitrogen, or sulphur bridges, and bear aliphatic, glucidic, aminoacidic and lipidic surface chains and chemically reactive functional groups (mainly acidic, e.g., carboxylic and phenolic, but also alcoholic hydroxyls, carbonyls, etc.) e.g., [30]. FAs generally feature structure and composition less complex than those of HAs, have a lower molecular mass and aromaticity and higher solubility, aliphatic character and content of O-containing functional groups. HA and FA are rich in hydrophilic and hydrophobic sites, exhibit a polydispersed and polyelectrolitic character, possess surface activity, present a relatively open, flexible, sponge-like structure rich of holes [31, 32], and contain a variable amount of highly reactive organic free radical moieties of prevalent semiquinonic nature [30]. All these properties qualify HS, and especially HAs, as privileged natural organic compounds in the interaction with organic contaminants.
The strength and stability of the interaction between organic contaminants and HS influence their persistence, immobilization and accumulation, mobility and transport, bioavailability and biotoxicity, degradability, volatilization and so on. In particular, HS are shown to be able to modify water solubility of organic contaminants, exert catalytic activity on some of them, act as photosensitizers in promoting their photodegradation, and, especially, adsorb them e.g., [33- 37]. Adsorption of contaminants onto HAs occurs through specific physical and chemical binding mechanisms, including ionic, hydrogen and covalent bonding, charge-transfer or electron donor-acceptor mechanisms, dipole-dipole and Van der Waals forces, ligand exchange, and cation and water bridging [33-37]. However, adsorption of nonpolar (hydrophobic) organic contaminants can be better described in terms of non-specific, hydrophobic or partitioning processes between the aqueous phase and the solid organic phase.
The role of HS in the phytoremediation of contaminated natural systems is well accepted but poorly investigated. Ke et al. [38] have shown a reduced ability of mangrove Kandelia candel (L.) Druce to remove pyrene with the addition of HA to contaminated sediments, as pyrene was more tightly bound to the organic matter. Recently published works demonstrated the fundamental control of natural organic matter on phytoremediation processes [39, 40].
PHYTOREMEDIATION OF AQUEOUS SYSTEMS FROM BISPHENOL A
In a recent study, Loffredo et al. [41] assessed the removal of BPA, at the concentrations of 4.6 mg L-1 and 46 mg L-1, from aqueous solutions during the germination and growth of eight herbaceous plants, the forage grasses couch grass Cynodon dactylon (L.) Pers., fescue Schedonorus arundinaceus (Schreb.), perennial ryegrass (Lolium perenne L.), Siberian wheatgrass Agropyron fragile (Roth) P. Candargy and white clover (Trifolium repens L.) and the horticultural species cucumber (Cucumis sativus L.), marrow plant (Cucurbita pepo L.) and radish (Raphanus sativus L.). Results obtained by these authors are shown in Fig. (1). The plant efficiency in BPA removal followed the order: radish > Siberian wheatgrass > fescue > perennial ryegrass = cucumber (more than 93 % of BPA removed) > marrow plant > white clover > couch grass at the lesser concentration of 4.6 mg L-1, and marrow plant > fescue > radish (more than 84 % of BPA removed) > perennial ryegrass > cucumber > Siberian wheatgrass > white clover > couch grass at the concentration of 46 mg L-1 (Fig. 1). Further, in the range of concentrations tested, BPA removal was proportional to the amount of compound present in the medium. Among the removal mechanisms, uptake by seedlings and degradation by seedling exudates seemed to play the most relevant role. Similarly, in a study with plant cell cultures of soybean Glycine max (L.) Merr., wheat (Triticum aestivum L.), foxglove (Digitalis purpurea L.), and thorn apple (Datura stramonium L.), Schmidt and Schuphan [42] attributed the rapid decrease of BPA concentration in the liquid medium to uptake by plant cells.
In other trials, BPA removal was assessed during plant growth in hydroponic culture [41]. In these experiments, the two plants perennial ryegrass and radish were grown for 8 days or 16 days in a nutrient solution added with the compound at concentrations of 4.6 mg L-1 and 46 mg L-1. After 8 days, only a reduced amount of BPA disappeared in the medium, with a maximum of 29% for radish at the lower BPA dose. Conversely, after 16 days perennial ryegrass was able to remove about 97% and 90% of BPA and radish removed 82% and 100%, respectively at the lower and higher concentration (Fig. 2). In these experiments, BPA removal was attributed to both plant uptake and degradation by co-present microorganisms stimulated by released plant exudates [41]. In a previous study conducted in hydroponic conditions, Ferrara et al. [43] found significant reductions of the compound in the BPA-contaminated growth medium of tomato and broad bean after a period of 21 days.
THE EFFECTS OF NATURAL ORGANIC MATTER ON PHYTOREMEDIATION OF ENDOCRINE DISRUPTORS BY HERBACEOUS SPECIES
In a very recent study, Gattullo et al. [39] assessed the removal of the endocrine disruptor 4-nonylphenol (NP) at a concentration of 1 mg L−1 by ryegrass (Lolium perenne L.) and radish (Raphanus sativus L.) during their germination. The decontamination process was evaluated in water only or in water containing two organic fractions simulating the organic content of real aqueous systems: a soil humic acid (HA) and a river natural organic matter (NOM) at concentrations of 10 and 200 mg L−1. At the end of germination experiments, NP in water only was not toxic for ryegrass and radish which removed, respectively, 37 and 51 % of the initial NP added. Similar results were obtained by Dettenmaier and Doucette [44] with crested wheatgrass and Domene et al. [45] with Lolium perenne and Brassica rapa. In water added with HA at the two doses, the removal of NP measured by ryegrass and radish was significantly higher than that found in water only, and this positive effect could be attributed to its stimulation of plant enzymatic activity, as previous shown by Nardi et al. [46] and Vaughan et al. [47]. When water was added with lower dose of NOM, the residual NP measured in the germination media of both ryegrass and radish was similar to that measured in water only. On the contrary, the addition of NOM at the higher dose caused significant and differentiated effects on NP removal by ryegrass (30 % increase) and radish (23 % decrease), with respect to water alone. The different compositional and functional properties of the HA and NOM fractions, in particular the phenolic groups content, might have had an important role in the biological activity. In the case of ryegrass, the stimulation could be ascribed to the same effects reported above for HA, whereas the reduced capacity of radish could depend on the ascertained phytotoxicity of NOM. According to a previous study, the simultaneous presence in the medium of an organic molecule and a humic fraction might cause synergistic negative effect on plant growth [48]. The disappearance of NP in the germination media could be ascribed to seedling activity along with a possible contribute by their associated microorganisms. In fact, Bokern et al. [49], in a study on the capability of root cultures of two Lupinus species to remove NP, found that most of the initial NP was absorbed by plant and partially transformed in bound residues, while a smaller amount of NP was absorbed and partially transformed or mineralized by microorganisms.
In another study, radish and ryegrass were tested for their capacity to tolerate and remove the combination of bisphenol A (BPA), 17α-ethynilestradiol(EE2), and linuron at the concentrations of, respectively, 1, 0.1 and 1 mgL-1 (first combination) or 10, 1, and 10 mgL-1 (second combination) from the following aqueous media: distilled water, a solution of natural organic matter (NOM) at a concentration of 20 mg L-1, a lake water and a river water [40]. Biometric measurements of seedlings at the end of germination experiments revealed that the phytotoxicity of the two combinations of EDs depended on the medium used.In particular, radish showed a discrete tolerance to the EDs in distilled water and lake water but it was inhibited in the NOM solution and river water. Ryegrass appeared negatively affected mainly in river water [40].
In the absence of seedlings, the three compounds did not significantly degrade in the four media with a maximum degradation of 5.8% for EE2 in both combinations. A marked removal of each compound was measured at the end of germination of both plant species except for linuron removal by ryegrass. In the experiments with radish, EDs removal followed the order: EE2 > BPA > linuron. In general, the medium did not significantly influence the removal capacity by this plant, even if the decontamination was less efficient in river water. With both ED combinations, ryegrass removed the three compounds in the order of BPA > EE2 > linuron. The amounts of BPA and EE2 removed by ryegrass were large and dependent on the type of aqueous medium, whereas the removal of linuron was the lowest and similar for the different media. Ryegrass showed generally a better decontamination capacity in lake water and distilled water rather than in NOM solution and river water. The residual amounts of BPA, EE2 and linuron measured in the different media at the end of phytoremediation experiments with radish or ryegrass were significantly lower than the initial amounts, but likely dangerous for aquatic organisms. It is reasonable to expect that an increase of plant biomass used in the phytoremediation could have improved phytoremediation. In this study, when the percentages of ED removed by ryegrass and radish were compared, apparently radish showed a better activity. However, when the comparison was made on the basis of µg of ED removed per g of fresh biomass, in general ryegrass was more efficient than radish in removing BPA and EE2 in both combinations. The monocotyledon ryegrass demonstrated a better decontamination performance than the dicotyledon radish. This is in agreement with other studies demonstrating that, among herbaceous plants, monocotyledonous species possess a higher phytoremediation potential for EDs than dicotyledonous ones [39, 41]. The principal mechanism of removal of BPA, EE2 and linuron was absorption and transformation by plants, whereas adsorption on root surface and temporary accumulation in plant tissues had a minor role [41, 50-52].
The influence of HA or NOM on phytoremediation of EDs has been also assessed in experiments using seedlings grown in hydroponics. Gattullo et al. [39] tested the capability of ryegrass and radish seedlings grown for 15 days in nutrient solution to remove NP at a concentration of 1 mg L−1. After 1 and 2 days of growth, their removal capacity was much higher than that observed in germination experiments, indicating that the decontamination efficiency increased with plant age. Imai et al. [53] also reported a complete removal of NP by Portulaca oleracea L. after 1 day of growth. The addition of 10 mg L-1 HA did not affect the removal efficiency of both species, whereas 200 mg L-1 HA reduced the removal efficiency of both species after 2 days. The addition of 10 mg L-1 NOM improved the ryegrass efficiency after 1 day, whereas 200 mg L-1 NOM reduced NP removal by radish after 2 days. Interactions of NP with the two organic fractions at the highest dose, may have resulted in a lower product bioavailability for plants and, therefore, a lesser removal by the same. At the end of experiments, only a little amount of NP was accumulated in plants, and even less in the presence of the two organic fractions, this may be due to a reduced plant uptake. Soares et al., [28] have suggested that the scarce accumulation of NP in plants could be related to its hydrophobic nature. In addition, according to Gonzàlez et al. [54] and Matsui et al. [55], NP similarly to other phenolic compounds could be bound to polysaccharides and proteins of cell walls on the root surface.
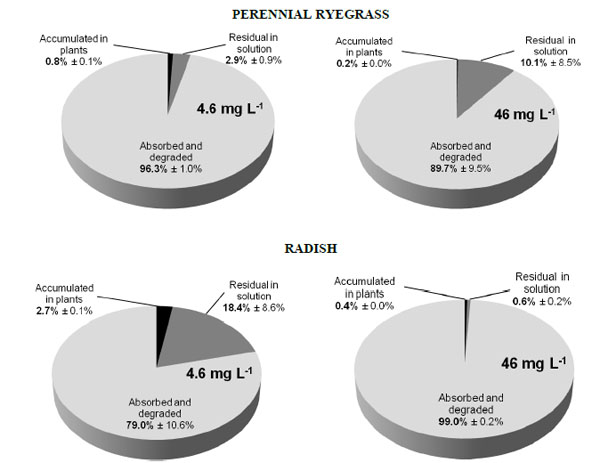
Distribution of BPA in plants and media after 16 days of growth of perennial ryegrass and radish in the presence of BPA at 4.6 mg L-1 and 46 mg L-1. [From Loffredo et al. [41] with permission].
In another study, Gattullo et al. [56] have tested the capability of the freshwater green alga Monoraphidium braunii (Nägeli) to remove BPA at concentrations of 2, 4, and 10 mg L-1, also in presence of NOM at concentrations of 2, 5 and 20 mg L-1. Generally, after 2 and 4 days BPA at lower concentrations was not toxic for the alga, whereas at the highest concentration it reduced algal growth and photosynthetic efficiency. A similar growth response was shown by the marine diatom Stephanodiscus hantzschii [57] and the green alga Chlorella fusca [58] which showed an evident toxicity at BPA concentrations equal or higher than 5 mg L-1 and 9 mg L-1, respectively. The sole NOM and its combinations with bisphenol A at the lower concentrations increased the cell number and the chlorophyll a content of algae, whereas all combinations between BPA at highest concentration and NOM resulted very toxic for the algae. After 4-day growth, good removal efficiency was exerted by M. braunii at concentrations of 2, 4 and 10 mg L-1 removing, respectively, 39%, 48% and 35% of the initial bisphenol A. NOM at any concentration scarcely influenced the BPA removal. In this study, M. braunii was more efficient than numerous different green algal species tested by Hirooka et al. [59] and Nakajima et al. [60] but less than Chlorella fusca [60]. It can be assumed that BPA was taken up by algal cells and successively accumulated and/or metabolized by these.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This work was funded by the University of Bari, Italy.


