RESEARCH ARTICLE
Preliminary Test of Candidate Rapid Diagnostic Test for the Detection of 38 kDa Mycobacterium Tuberculosis Antigen in Saliva
Tri Yudani Mardining Raras1, *, Nabila Rahmadani2, Maimun Zulhaidah Arthamin3, Muhammad Rizki1, 4, 5
Article Information
Identifiers and Pagination:
Year: 2024Volume: 18
E-location ID: e18740707277154
Publisher ID: e18740707277154
DOI: 10.2174/0118740707277154240108062155
Article History:
Received Date: 20/09/2023Revision Received Date: 29/11/2023
Acceptance Date: 18/12/2023
Electronic publication date: 12/1/2024
Collection year: 2024

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background and Objectives
Identifying tuberculosis (TB) in pediatric cases is a major challenge in developing countries, as children have problems with expelling sputum, making specific diagnostics crucial. The objective of the study was to develop a rapid test using polyclonal antibodies to detect antigen 38kDa from Mycobacterium tuberculosis in the saliva of TB patients.
Materials and Methods
The recombinant protein Ag38 was purified using the Ni-NTA purification kit. Polyclonal antibodies were generated in BALB-c mice using 50 µg/ml of purified Ag38 recombinant protein. A Lateral Flow Assay (LFA) was constructed, employing 5 mg/mL colloidal gold-labelled polyclonal antibody anti-Ag38 in the test line to capture the conjugates, while goat anti-mouse IgG was used in the control line. The LFA was tested in 5 TB patients and 7 healthy person served as negative control .
Results
The recombinant protein achieved 95% purity. The rapid test kit, with a detection limit of 5.3 µg/mL, successfully identified Ag38 protein in TB patient saliva (positive control) and not in healthy human serum (negative control). While reproducibility was confirmed for TB patients, results were inconsistent for healthy individuals.
Conclusion
The Lateral Flow Assay using polyclonal antibody Ag38 displays promise in detecting M tuberculosis antigen in the saliva of TB patients. Further validation with more TB patient saliva samples is needed to determine LFA sensitivity and specificity.
1. INTRODUCTION
Despite advancements in TB treatments, pediatric cases persist globally, notably in resource-limited nations like Indonesia. Pediatric TB does not have specific symptoms like adult TB, and children face difficulty in producing sputum, which makes the “gold standard” culture examination impossible [1-3]. The WHO recom-mends Xpert MTB/RIF for pulmonary TB symptoms as an initial diagnostic test in children [4]. However, cost and expertise hinder its widespread use. This is particularly problematic for children unable to produce sputum on demand and for cases of extra-pulmonary TB [5, 6].
Given the aforementioned factors, there is a need for an innovative diagnostic tool facilitating the direct on-site ex vivo detection of TB infection in children. LFA ist a robust point-of-care (POC) technique ideal for resource-limited areas due to their rapid detection, minimal sample requirement, and cost-effectiveness, making healthcare more accessible, especially in low-income communities [7]. LFAs employ electrochemical lateral flow strips [8], utilizing an immunochromatographic process on a nitrocellulose membrane. The antigen-antibody reaction produces a color band from attached gold particles [9]. There are successful cases of LFAs detecting Mycobacterium tuberculosis (Mtb) [10, 11].
Salivary samples, being non-invasive, cost-effective, easily stored, and requiring no specialized personnel, are ideal for this purpose [12-14]. Saliva, containing mucosa linked to the respiratory tract, which is the primary Mtb infection route, serves as a valuable information source regarding mucosal-level events [5, 12, 15]. Our prior study demonstrated that the partially purified Ag38 protein detected elevated sIgA levels in TB patients’ saliva, distinguishing them from non-TB adults [16].
The 38-kDa protein, among other antigens, is valuable for TB diagnosis. This protein is actively secreted in mycobacterial cultures, whereas host immune responses are target proteins secreted by Mtb. This specific protein induces significant cellular and humoral immune responses in human TB, providing protective immunity in animals and humans [17]. Bioinformatic analysis revealed this protein contains two epitopes that recognize B-cells [18]. Arjuno et al. assessed salivary secretory IgA reactivity to the 38 kDa antigen, indicating that this antigen harbors a specific immunodominant epitope against Mtb virulence. Hence, Ag38 kDa can be used as material for TB diagnostic test kits [19]. The antibody response to the Mtb antigen varies among individuals [20]. In the previous study, we succeeded in expressing 38 recombinant antigens (Ag38-rec) of Mtb in Eschericia coli [21].
In this study, we aimed to overexpress and purify the Ag38-rec protein and generate polyclonal antibodies in mice. Mice were chosen due to the limited protein yield achievable with the purification kit. The obtained antibody will be used to develop a cost-effective TB immunodiagnostic tool for Ag38 antigen detection in saliva. It will serve as the key element in a LFA-based rapid diagnostic test (RTD). Our hypothesis is that the polyclonal antibody can recognize Ag38 protein from Mtb in the saliva of TB patients.
2. MATERIALS AND METHODS
2.1. Overexpression and Purification of Protein Ag38 kDa
The Ag38 kDa-rec protein was overexpressed following a prior established method [21]. The Ag38-rec was purified using a Ni-NTA kit that followed the manufacturer’s procedure (Protino, Duuren, Germany). Monitoring of protein purity was carried out using the SDS-PAGE method [22]. To increase the protein purity, the band corresponding to 38 kDa in SDS-PAGE gel was cut and electro-eluted.
2.2. Mouse Immunization and Polyclonal Antibody Collection
A polyclonal antibody was produced in BALB/c mice. Ten female mice (n=10) 6-8 weeks-old mice were purchased from PT Indo animal Lab (Bogor, Indonesia). The Ag38-rec protein (25 μg/100 μl) was emulsified using Freund’s complete adjuvant (1:1) and injected subcutaneously. Booster injections were carried out in the second, third, and fourth weeks using antigen emulsified with incomplete Freund’s adjuvant. To obtain polyclonal antibodies, whole blood from mice was aspirated from the heart, then the serum was separated by sentrifugation at 2500 g. The supernatant was mixed with Ammonium sulfat 25% and stirred slowly at 4°C overnight. Following centrifugation at 5000 g at 4°C for 10 min, the pellet was diluted with PBS for dialysis overnight. After centrifugation at 10.000 g for 10 min, the supernatant was stored in -20 °C [23].
2.3. Measurement of Antibody Level using ELISA Method
The frozen serum antibody was thawed at RT. A total of 50 μL of Ag38-rec antigen was coated in ELISA plate wells and incubated overnight at 4°C. On the next day, it was washed with phosphate-buffered saline (PBS), blocked using 1% BSA in 50 μL of PBS and incubated for 3 hours at RT. After washing with PBS, 50 μL of peroxidase-conjugated anti-mouse IgG (Invitrogen, USA) was added and incubated overnight at 4 °C. Post washing, 50 μL of the substrate, 3,3',5,5'-Tetramethylbenzidine (TMB) (Thermofisher, USA) was added. Finally, the reaction was terminated using a stop solution. The plate was read on an ELISA reader at a wavelength of 450 nm.
2.4. Preparation of Lateral Flow Immunochromato-graphic Assay Strip
Colloidal gold was prepared as follows: 50 mL of 0.1 g/L chloroauric acid solution was brought to a boil with continuous stirring (100 rpm). Two mL of 1% w/v trisodium citrate solution was added and stirred continuously for 6 minutes until the solution turned wine red. It was then cooled to RT and stored at 4°C [24].
The preparation of colloidal gold-labeled antibodies [25] is as follows: 10 mL of the previous colloidal gold solution (pH 8) using 0.1 M K2CO3 and 0.1 M HCl). With gentle stirring, 0.1 mL of 2.5 mg/mL anti-Ag38 antibody was added drop by drop, followed by 1 mL of 5% BSA (g⁄v) and the solution was stirred for 30 minutes. After centrifugation at 3500 g for 15 minutes, the supernatant was further centrifuged at 1300 g for 40 minutes. The bottom solution (antibodies labeled colloidal gold) was taken and washed using 0.02 M phosphate buffer pH 7.4 (containing 5% sucrose, 1% BSA, and 0.5% PEG 6000). The conjugated product was reconstituted to 1 mL using 0.02 M phosphate buffer containing 0.02% NaN3 and finally stored at 4 °C.
Immunochromatographic strips were prepared using 5 mg/mL polyclonal anti-Ag38 for the test line and goat anti-mouse IgG for the control line. The components were sprayed onto the nitrocellulose membrane and conjugate pad of an immunoassay card (Ahlstrom 6613, Finland), followed by drying at RT for 1 hour [26]. Cards were cut into 4 mm strips using a strip cutter.
2.5. Kit Assembly
Strips were inserted into a cassette housing and assembled into a ready-to-use rapid test kit, each individually stored in desiccant-filled aluminum foil at 4°C.
2.6. Specimens Test
The rapid kits were subsequently tested with 5 μL solution containing Ag38-rec protein with concentration of 5.3 μg/mL as positive control and healthy human serum as negative control. The kits successfully identified the Ag38 recombinant protein in the positive control samples.
2.7. Clinical Specimens
Saliva samples (n=5) were collected in October 2022 from 5 pulmonary adult TB patients at the Islamic Hospital in Malang, Indonesia, and 7 healthy volunteers from medical students at the Faculty of Medicine, Brawijaya University. Tuberculosis diagnosis involved a respiratory medicine specialist's clinical examination, chest X-ray, and PCR examination. Additional inclusion criteria were TB patients who had not undergone therapy. Informed consent was obtained from both patients and healthy individuals before specimen collection.
3. RESULTS
3.1. Purification of Protein Ag38-rec
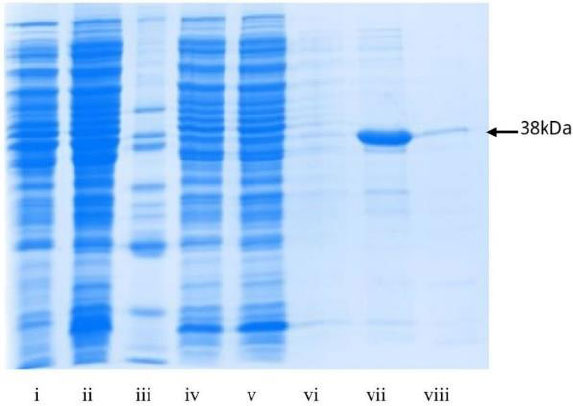
Ag38-rec antigen was successfully expressed in E coli BL21-(DE-3) and then purified using the Ni-NTA kit IMAC method (Fig. 1i-viii).
The antigen underwent Western Blot verification against the monoclonal antibody anti-Ag38 (data not shown) before being induced into mice [27].
3.2. Detection of Polyclonal Antibody
Results of antibody measurement on days 10, 27, and 37 were 46.20 mg/ml, 46.77 mg/ml, and 47.94 mg/ml, respectively. This antibody was forwarded to the Hepatitis Laboratory, Mataram, as the primary material for LFA production. The titer of polyclonal antibody from the ELISA test demonstrated that the Ag38-rec antigen has low immunogenicity (Tables 1). While antibody concentration increased post-antigen induction, it lacked statistical significance.
| Group | Antigen (mg/ml) | Antibody (mg/ml) | ||
|---|---|---|---|---|
| 0.25 | 0.5 | 1 | ||
| Before vaccination | 2.5 5 10 |
1.282 1.444 1.454 |
1.225 1.338 1.42 |
1.236 1.366 1.365 |
| Booster 1 | 2.5 5 10 |
1.443 1.124 1.404 |
1.382 1.082 1.380 |
1.303 1.256 1.303 |
| Booster 2 | 2.5 5 10 |
1.302 1.178 1.327 |
1.212 1.224 1.250 |
1.302 1.288 1.271 |
| Booster 3 | 2.5 5 10 |
1.214 1.185 1.382 |
1.157 1.227 1.324 |
1.237 1.306 1.406 |
3.3. Assembly of LFA to Detect Mtb Antigen Ag38 in Saliva
The LFA was assembled in collaboration with Hepatitis Laboratory Mataram, Indonesia. The antibody coated on the LFA kit showed that they could recognize Ag38-rec antigen with a minimum concentration of 5 μg/ml, while the polyclonal antibody with a concentration of 2.5 μg/ml in the conjugate (Fig. 2).
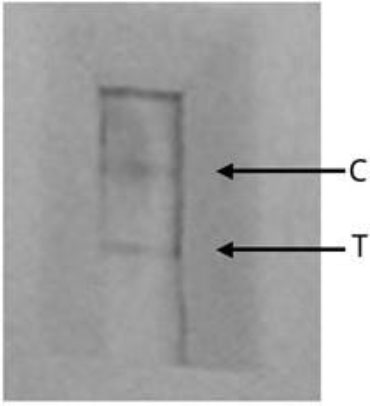 |
Fig. (2). LFA prototype with anti-Ag38 rec polyclonal antibody; C- control, T-test line. |
| No. |
Saliva (µl) |
H2O (µl) |
Buffer (µl) |
Condition | Time of Stripe Appears (min) |
|---|---|---|---|---|---|
| 1 | 40 | - | 40 | dry | 20 |
| 2 | 20 | - | 40 | dry | 20 |
| 3 | 20 | - | 20 | dry | 20 |
| 4 | 20 | 20 | 40 | dry | 20 |
| 5 | 30 | 10 | 40 | dry | 20 |
| 6 | 20 | 20 | 40 | dry | 20 |
| 7 | 10 | 30 | 40 | 2 stripes appeared | 5 min |
| 8 | Mixed 30 ml H2O and 10 ml saliva | 40 | 2 stripes appeared | 5 min | |
| 9 | Mixed 20 ml H2O and 20 ml saliva | 40 | dry | 20 min | |
No 6 and 7 dripping was done from H2O, saliva and buffer.
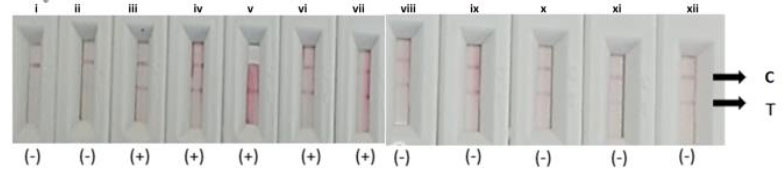 |
Fig. (3). Detection of Ag38 antigen in adult TB patients’ saliva using LFA prototype (iii-vii) compared to healthy individuals’ saliva (i-ii, viii-xii). (+) C- control line, T- test line. |
The LFA saliva test followed the kit manual: 40 ml saliva was dripped into the cassette hole, followed by 8 drops (40 ml) of 1% casein in PBS buffer, and observed for 15-20 minutes. Unexpected strip absence led to saliva dilution with H2O. After various dilutions were tested, the optimal conditions were obtained (Table 2).
3.4. Trial of Lateral Flow Assay on TB Patient
Upon completing the prototype, it was tested on the saliva of 5 adult TB patients, yielding positive results with 2 strips appearing in 5-10 minutes. However, testing on 7 healthy individuals as a negative control showed inconsistent performance, with 4 out of 7 kits displaying 2 strips, indicating the need for specificity evaluation (Fig. 3).
4. DISCUSSION
The challenges in diagnosing pediatric TB patients in developing countries emphasize the need for a precise diagnosis beyond sputum [28]. The development of RTD for detecting TB antigens in saliva has significant implications for pediatric populations in resource-limited settings [15, 29].
While numerous rapid tests focus on antibody detection, only a limited number can identify antigens in samples. One study claimed the use of LFA to detect antigen of Mtb in saliva with high sensitivity and specificity. It could be due to the combined antigen they used, i.e., CFP10-ESAT6, instead of the single antigen we used [11]. Detection of TB antigens using a specific antigen detection kit can provide direct evidence of infection and assist in the early initiation of treatment [30]. The Ag38 protein, among the proteins secreted early in infection, holds promise as an effective initial screening tool for tuberculosis diagnosis.
Our RTD for early pulmonary TB detection is capable of identifying Ag38 antigen presence in just 10 µl of saliva. Initially, we tested the LFA on adult TB patients, characterizing the response of polyclonal antibodies against the Ag38 antigen in their saliva. These results, while preliminary, indicate the need for optimization. Nevertheless, the LFA's performance is comparable to that using polyclonal antibodies generated from the CPF10-ESAT6 fusion of Mtb [11]. Therefore, despite the simplicity of the present rapid test procedure, it is hoped that our LFA can differentiate between pulmonary TB patients and healthy people, particularly among pediatric TB patients. To increase the detection limit, enhancing antibody purity and minimizing antibody concentration is very crucial [24].
Saliva samples from TB patients consistently yielded positive results on the LFA, affirming the recognition of Ag38 by the polyclonal antibody. Notably, the concentration of the polyclonal antibody on the LFA (2 µg/ml) remained relatively high, potentially attributed to the nature of polyclonal antibodies. In comparison, the monoclonal antibody used by Seele employed only 0.064 µg [10]. Transitioning to monoclonal antibodies could enhance antibody specificity [24].
The inconsistency in LFA results among saliva samples from healthy controls may arise from true positives indicating asymptomatic Mtb infection or false positives. False positives in salivary Mtb antigen detection tests could result from cross-contamination with other Mtb antigens or pathogens, especially non-M. Tuberculosis mycobacteria. Since polyclonal antibodies recognize multiple epitopes, there is a risk of cross-reactivity with diverse targets [31]. Positive results in healthy controls might be due to asymptomatic TB infections, given the endemic nature of TB in our country [32]. Pre-analytical factors, such as saliva content (e.g., eating before the test and collection time), also play a part. Saliva analysis susceptibility to variations based on sampling technique influences antigen identification on the LFA cassette [33].
The initial study indicates the developed RDT's potential for early diagnosis, disease progression monitoring, and treatment response. Despite these prospects, recognizing limitations like antigen concentration variability, immune response, and individual differences in saliva composition is crucial. Further research is needed to refine RDT performance. Notably, due to time constraints, strict inclusion criteria were not implemented in this phase.
Finally, TB RTD results with saliva samples mark a notable advancement in TB diagnosis, especially in pediatric and resource-limited settings. The RDT's non-invasive, cost-effective, and patient-friendly attributes offer promise for enhancing TB management. Subsequent studies are essential to validate these findings, assess diagnostic performance, evaluate antibody kit monoclonality, explore real-world applicability, integrate them into healthcare systems, and assess the overall impact on TB diagnosis and patient outcomes. Possible reasons for diminished immune response include mice's unsuitability and suboptimal antigen induction procedures in the Ag38 context.
CONCLUSION
We developed a rapid test using lateral flow immunoassay utilizing polyclonal antibody produced from local strain Mtb Ag38-rec antigen. The lateral flow assay successfully detected the presence of Ag38 antigen in the saliva of a TB patient in five minutes. Nevertheless, the sensitivity still needs to be improved.
ABBREVIATIONS
| TB | = Tuberculosis |
| LFA | = Lateral Flow Assay |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All experimental protocols have been proven by the Universitas Brawijaya Ethics Committee (NO: 081-KEP-UB-2022).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013. All the methods involved in animals experiments an reported in accordance with the ARRIVE guidelines.
All the reported experiments were in accordance with The ARRIVE guidelines and the US National Research Council's “Guide for the Care and Use of Laboratory Animals”.
CONSENT FOR PUBLICATION
Informed consent was obtained from both patients and healthy individuals before specimen collection.
AVAILABILITY OF DATA AND MATERIALS
All data in this study are presented in this article.
FUNDING
This project was financially supported by the research grant PNBP 2022 from the Faculty of Medicine, Universitas Brawijaya.
CONFLICT OF INTEREST
We declare that there is no conflict of interest in this study
ACKNOWLEDGEMENTS
We appreciate Suci Megasari, MP, for technical support and Prof Mulyanto from Hepatitis Lab, Mataram, for valuable discussions in rapid test construction. Special thanks to Unisma Hospital and Dr. Iin Chozin for patient screening.