REVIEW ARTICLE
Nanomaterials as Protein, Peptide and Gene Delivery Agents
Anika Guliani1, 2, Amitabha Acharya1, 2, *
Article Information
Identifiers and Pagination:
Year: 2018Volume: 12
First Page: 154
Last Page: 165
Publisher ID: TOBIOTJ-12-154
DOI: 10.2174/1874070701812010154
Article History:
Received Date: 25/4/2018Revision Received Date: 19/5/2018
Acceptance Date: 11/6/2018
Electronic publication date: 29/08/2018
Collection year: 2018

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Nanomaterials offer significant advantages in delivery of different biomolecules which suffer from drawbacks like poor bioavailability, low stability and retention time, degradation in biological systems etc. Nanotechnological approach has shown promising results for the sustained release of these biomolecules with minimal toxicity concerns. The present review describes a comprehensive outlook of the different nanomaterials used for the delivery of these biomolecules.
Methods:
Current literature reports related to protein, peptide and gene delivery agents have been reviewed and classified according to their applications.
Results:
Studies suggested that the nanomaterial based delivery agents can be broadly classified in to five categories which include metallic NPs, polymeric NPs, magnetic NPs, liposomes and micelles. All these materials provided significant improvement in the targeted delivery of biomolecules.
Conclusion:
Concerns regarding the bioavailability, stability and delivery of proteins, peptides, genes need to be investigated to improve their therapeutic potential in the biological milieu. The use of nanoparticles as drug delivery vehicles may avoid undesirable hazards and may increase their pharmaceutical efficacy.
1. INTRODUCTION
Proteins and peptides are long being used to treat a wide variety of human diseases. Proteins play an irreplaceable role in life, taking part in all vital life activities at the molecular level. There are different varieties of proteinaceous molecules which act as antibodies, growth factors, antigens and possess excellent antioxidant, antibacterial, antiviral activities, provide support for cholesterol synthesis and boost immune system [1]. But factors like aggregation, unfolding/misfolding, small size etc. restrict the therapeutic potential of these biomolecules [2, 3]. Likewise, the delivery of genes to the organs is also considered as one of the major issues since this can lead to enhanced expression of the already present genes. Gene delivery has proven to be effective in the repair of many tissues by gene knockdown method. It has been also proved to be beneficial in causing apoptosis of the damaged cells by silencing the mRNA produced and inhibiting the production of the functional protein. The conventional methods for the transfection are associated with the use of viral vectors which have several disadvantages viz., high manufacturing cost, mammalian cells compatibility issues, opsonization by body cells, increased immunogenic responses, etc. [4-7]. Nanomaterials can be used for the oral delivery of the proteins, peptides and genes, since these offer advantages viz., increase the solubility of poorly water-soluble molecules, help in targeting gastrointestinal tract, allow transcytosis across the mucosal layer, protect encapsulated biomolecules from adverse environmental conditions, etc. [8]. Nanoparticles (NPs) being small in size and highly biocompatible in nature, allow slow and sustained release of biomolecules which can cross the epithelial barrier and provide effective therapeutic outcomes with minimal dosage. The delivery of the genes with the help of non-viral vectors is now easy since these are biocompatible, non-immunogenic in nature. There are a number of gene carriers which have been designed on the basis of nanotechnology and have proven to be capable of delivering the cargo at the targeted sites with minimal toxicity concerns [9]. This review will cover a description of different protein, peptide and gene delivery agents along with their potential therapeutic applications.
2. NANOMATERIALS FOR PROTEIN AND PEPTIDE DELIVERY
Here in this section, different classes of nanomaterials used for protein and peptide delivery have been discussed.
2.1. Metallic Nanoparticles
Metallic NPs like gold (Au), silver (Ag), copper (Cu), etc. have been used for the delivery of various proteins and peptides. In the literature, gold nanoparticles (AuNPs) have been used for nucleus targeting by functionalizing their surface with transactivator of transcription (TAT) peptides [10, 11]. In general, peptide conjugated NPs were synthesized by immobilization of thiol groups of the peptide on the AuNPs surface. Bovine Serum Albumin (BSA) and streptavidin have been covalently conjugated on the AuNPs surface [10, 12]. AuNPs passivated with poly-ethylene-glycol (PEG) were functionalized with peptide-toxin conantokin-G. These functionalized NPs can selectively bind to N-methyl-D-aspartate (NMDA) receptors present on the neuron surface and have been found to block the cell death pathways [13]. AuNPs attached with amyloid growth inhibitor peptide (-CLPFFD-NH2-) showed an easy recognition of murine bone marrow macrophages in comparison to the bare AuNPs. These peptide conjugated NPs showed enhanced macrophage response by secreting more inflammatory cytokines like TNF-α, IL-6 [14]. Cecropin-melittin, an Antimicrobial Peptide (AMP) (KWKLFKKIGAVLKVLC) containing cysteine amino acid at its terminal end, was conjugated with AuNPs. The conjugated AuNPs exhibited low polydispersity and narrow size distribution of 14 nm and was tested against S.aureus and E.coli for their antibacterial activity and in vivo studies. Results suggested better antibacterial activity for peptide conjugated AuNPs [15]. Another AMP, esculentin-1a has been conjugated to AuNPs with the help of PEG and has been found to be effective against P. aeruginosa at a low concentration. The conjugated NPs were non toxic to human keratinocytes and could break the bacterial membrane easily [16]. An AMP odorranain-A-OA1 (OA1) (VVKCSYRLGSPDSQCN) was conjugated to citrate-capped silver nanoparticles (AgNPs) (size of ~12-14 nm). The peptides attached to AgNPs were found to be more stable and displayed better antibacterial activity as compared to the native peptide or the bare AgNPs. The conjugates were also less toxic to the mammalian cells [17]. Breast cancer cells (MCF-7) have been treated with AgNPs attached with Cell Penetrating Peptides (CPP). The CPP aided in enhanced intake of AgNPs across the cellular membranes, thereby causing the cancer cell death [18]. The effect of AgNPs along with MBP-1 peptide was studied on the disease-causing bacteria S.aureus which is commonly resistant to many antibiotics. The wound caused by the bacteria has been found to heal and the bacterial colonization also decreased at the wound site due to mutual effect of both the AgNPs and MBP-1 peptide [19]. The specific peptide sequence of arginine, glycine, aspartic acid (RGD) interacts with integrin receptors in various adhesion processes for cell targeting. Fluorescent peptide RGD was immobilized on AgNPs to enhance the NP entry into the leukemia (α5β1) and neuroblastoma (αvβ3) cells overexpressing their specific integrins [20]. Silicon NPs (SiNPs) conjugated with RGD have been effectively used as a tracking agent as well as for the killing of the specific malignant cancer cell U87MG [21]. SiNPs coated with bovine serum albumin/ human serum albumin (BSA/HSA) proteins have been studied for cholesterol effluxing as well as for fluorescence imaging in HUVEC and HCAEC cell lines. The HSA coated SiNPs have been reported to show enhanced permeation as well as cholesterol effluxing in both the cells [22]. A report on the functionalization of the SiNPs has described conjugation of SiNPs with chitosan undecylenic acid which was further modified with L-cysteine and CPP. These modified L-cysteine NPs have been found to be effective for the oral delivery of the insulin and have also been found to increase the bioavailability of insulin by ~ 1.86 times as compared to bare NPs [23]. Extracellularly synthesized copper nanoparticles (CuNPs) by stem latex of Euphorbia nivulia have been exploited for in vitro administration of NPs in A549 cancer cell lines. CuNPs were found to be toxic depending upon the dose while the latex coated CuNPs have been found to be non toxic and have been used as a delivery agent in the cancer cell lines [24]. Table 1 summarizes reports of peptide conjugated or encapsulated metallic NPs along with their specific applications.
| Metallic NPs | Peptide Sequence | Functions | References |
|---|---|---|---|
| AgNPs | Odorranain-A-OA1 (OA1) (VVKCSYRLGSPDSQCN) |
Antimicrobial peptide | [17] |
| AgNPs | CRGDKGPDC | Increased penetration in the glioma cells | [78] |
| AgNPs | CSG-LL37 | Antimicrobial activity, wound healing, neovascularization and angiogenesis | [79] |
| AgNPs | GGGRRRRRRYGRKKRRQRR (G3R6TAT) | Cell penetrating peptide, reducing agent and surfactant | [80] |
| AgNPs | Ac-LIVAGK-NH2 | Antibacterial | [81] |
| AuNPs | CALNN–AALRRASLG | cAMP-dependent protein kinase A | [82] |
| AuNPs | Ac–Cys[Ac]–Gly–Dphe–Pro–Arg–Gly–Cys[Ac] –OH | Substrate for thrombin | [83] |
| AuNPs | KWKLFKKIGAVLKVL | Antibacterial | [84] |
| AuNPs | Pro-His-Cys-Lys-Arg-Met; Pep-A | Antioxidant | [85] |
| AuNPs | LA-WKRAKLAK | Anticancer | [86] |
2.2. Polymeric Nanoparticles
The use of polymeric NPs has also been extensively studied for encapsulation, attachment of various proteins/peptides for targeted delivery, increase the bioavailability, retention time, stability, etc. of the attached biomolecules. Polymers like poly (lactic-co-glycolic acid) (PLGA), chitosan, poly (lactic acid) (PLA), polycaprolactone (PCL) have been used for the attachment of different proteins and peptides. Lysozyme and a transforming growth factor (TGF-β) have been encapsulated into PLGA polymer. The encapsulated proteins were found to be more stable; their structural integrity as well as the biological activity were also found to retain in the encapsulated form [25]. Many enzymes degrade when administered into the body and thus are unstable in nature. The enzyme laccase was attached on the surface of chitosan NPs and found that the enzyme sensitivity at different pH and temperature was similar to that of the free enzyme. The enzyme was found to be more stable and showed enhanced activity towards degradation of microbes than the free enzyme [26]. The surface of PLA NPs was modified with Polyethylene Glycol (PEG) and to this an anticancer peptide NuBCP-9 was encapsulated. The spacer length of PEG and the molecular weight of PLA varied which aided in more effective delivery of NuBCP-9 and caused apoptosis of the cancer cells [27]. Another polymer (poly(d,l lactic-cohydroxymethyl glycolic acid) (pLHMGA) has been used for the preparation of vaccines against the cancer cells. A long synthetic peptide isolated from HPV16 E7 oncoprotein together with toll like receptor 3 has been encapsulated into the NPs and were found to be effective in decreasing the tumor and suggested no adverse effects on the body as compared to the free adjuvants administered with vaccines [28]. Similarly, BSA was encapsulated into a PLGA with an encapsulation efficiency of 65%. It was observed that the concentration of the polymer used was directly proportional to the BSA encapsulation efficiency [29].
2.3. Liposomes
Liposomes act as good carriers for both water-soluble as well as water-insoluble molecules. A liposome-based drug delivery system which can cross the Blood Brain Barrier (BBB) has been developed. The surface of the PEG-modified liposomes was further conjugated with Angiopep-2 peptide. This peptide acted as a targeting ligand and could bind with bEnd.3 cells and can cross the BBB when injected intravascularly into the body. These liposomes have been further checked for their ability to entrap ultrasound contrast gases for bioimaging applications [30]. Mannosylated liposomes have been used as an encapsulating agent for MBP peptides. These peptides could retard the growth of encephalomyelitis. These liposomes have been administered into the body of multiple sclerosis patients where they have been found to be useful in curing the disease by decreasing MCP-1/CCL2, MIP-1β/CCL4, IL-7, and IL-2 factors in the serum of the patients [31]. Again, DAMGO(H-Tyr-D-Ala- Gly-MePhe-Gly-ol), an opioid peptide with poor brain penetrating properties have been successfully encapsulated in glutathione (GSH)-coated liposomes. The biological activity was found to enhance in comparison to the empty GSH-PEG liposomes and the corresponding results suggested that the PEG liposomal DAMGO has been efficient in targeting brain without the use of any specific ligand [32]. Hydrophobic polyampholyte-modified liposomes, synthesized using a combination ofε - poly-L-lysine- dodecylsuccinic anhydride- succinic anhydride were compared with unmodified liposomes for the delivery of lysozyme. The polyampholyte- modified liposomes exhibited high encapsulation efficiency, stability and low cytotoxicity. These liposomes have been found to bypass the endocytic pathway and thus serve as a very efficient tool for delivery of the protein [33]. An antidiabetic peptide from Atlantic salmon (Salmo salar) protein hydrolysates (SPH) has been loaded into liposomes. The surface of the liposomes was coated with chitosan which enhanced the stability and could preserve the SPH loaded liposomes even at 4°C [34]. The induction of adaptive immune response by a subunit vaccine, encapsulated in liposomes, with the iNKT adjuvant α-GalCer, has been found to be responsible for the enlarged production of IFN-γ and cytotoxic T-cell responses. The vaccines administered intravenously induced the anti-tumor responses and caused immune stimulation [35]. BSA has been encapsulated in to pH responsive polymer based liposomes. These liposomes when administered in the rat bladder at pH 6.5, showed better delivery of the loaded protein at the bladder site of MB49 cells and macrophages as compared to the physiological pH (7.4) [36]. The cationic AMPs play vital roles to fight against several pathogenic bacteria as well as these are helpful in innate immunity response. But these lack stability, infer cytotoxicity and are rarely available to the body. To increase the bioavailability and retention time, two cationic AMPs viz., LL-37 and indolicidin, have been encapsulated into liposomes and tested against herpes simplex 1 virus. The LL-37 formulation formed ~110 nm sized liposomes which were found to be more stable.These nanoformulations were efficiently taken up by HaCaT cells and showed decreased cytotoxicity level compared to the free peptide (Fig. 1) [37].
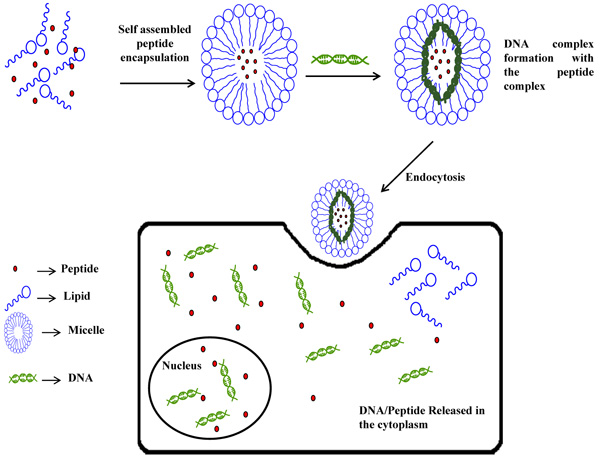 |
Fig. (1). Nanoencapsulation of peptides and DNA into liposomes. |
2.4. Micelles
Albumin, a negatively charged protein found in blood, has been used as biocompatible material for the encapsulation of proteins like lysozyme, Spry 1, etc. The surface of the albumin protein was modified with poly(oligo (ethylene glycol) methyl ether methacrylate) which enhanced the stability of the NPs formed. This PEGylated albumin polyion complex micelle has been found to bind with cationic polymers and also retained the functional aspect of the encapsulated molecule. The Spyr 1 showed better anticancer activity in MDA-MB-231 and MCF-7 cell lines, by hampering the growth of three dimensional multicellular tumor spheroids [38]. In order to achieve higher drug payload at the tumor sites, the surface of the doxorubicin (Dox)-loaded DSPE–PEG micelles have been modified with GE11 peptide which helped in the targeted delivery of Dox at the tumor site [39]. Cyclic peptide RGDfK can effectively bind with αvβ3 which are overexpressed on the cancer cells. The strong affinity resulted from the presence of carboxylate, guanidinium, and hydrophobic groups of c(RGDfK). In this respect, c(RGDfK)-PEG-PLA/PEG-PLA/DTX has been prepared which showed strong potential to act as anti-cancer agents to the tumor cells,over expressing αvβ3 on the surface [40]. The fatty acid was covalently attached to the surface of proteins which further served as an anchoring platform for plasma membrane binding. Reverse micelle approach has been used to encapsulate two myristoylated proteins namely recoverin and HIV-1 and was found to retain their functional aspects [41]. The reverse micelles of polyurathene have been loaded with BSA, with and without heptakis(2,6-di-O-methyl)-b-cyclodextrin (DM-b-CD) conjugation. The BSA encapsulation efficiency, release rate was found to be significantly higher for the DM-b-CD-containing micelles [42]. Reverse micelles have also been reported to improve the stability and half-life of the lipase from R. delemar and C. rugose encapsulated in to it [43]. PLA micelles loaded with Dox and surface coated with PEG have been evaluated against Caco-2 cell. The micelle were attached with a twelve amino acid peptide, TWYKIAFQRNRK, which had a strong binding affinity for α6β1 receptor, abundantly expressed on Caco-2 cells [44].
2.5. Magnetic Nanoparticles
The superparamagnetic iron oxide NPs (SPIONPs) have been synthesized and their derivatization was done using a TAT-derived peptide Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-Gly-Tyr-Lys(FITC)-Cys-NH2.These NPs were found to enter the hematopoietic and neural progenitor cells at a concentration of 10-30 pg of iron per cell. These NPs were also found to be present in the cytoplasm of CD34+ cells and showed no cytotoxic effects [45]. SPIONPs have been surface modified with PEG and was used as MRI contrast agent. These were further attached with TAT for quick cellular internalization [46]. The surface of IONPs has been coated with PEG,followed by further functionalization using heparin at a concentration of 35.4 μg of heparin/mg of Fe. These NPs have been found to bind with protamine with loading capacity of 22.9 ± 4.7 μg/mg of Fe. Again, these NPs showed better retention ability in the blood and accumulated in a smaller amount in the liver, suggesting their easy recognition by the immune system [47]. The magnetic NPs coated with 2,3 –dimercaptosuccinic acid have been covalently attached with a GEBP-11 peptide (GEBP11-DMSA-MNPs)via amide bond coupling reaction. Further, these were attached to Cy5.5 via ester bond formation. Cell lines viz., HUVEC and SGC7901 were used to evaluate the magnetic as well as fluorescence imaging potential of the prepared NPs. The GEBP-11 peptides aided in higher uptake of the NPs and no cytotoxic effects were found. These GEBP11-DMSA-MNPs have been used as an imaging probe for evaluating the angiogenesis in gastric cancer cells with high specificity and sensitivity [48]. The monocrystalline IONPs have been attached with TAT peptide (GRKKRRQRRRGYK) which helped in the internalization of the IONPs and allowed magnetic resonance imaging of the targeted site. The biodistribution studies have been done using BalB C mice by injecting these NPs and major accumulation was found in lymph, spleen and liver [49]. To increase the intracellular effectiveness of IONPs, TAT peptide (YGRKKRRQRRR) has been attached to IONPs. The effect of these NPs has been studied in lung cancer cell lines viz., A549 and H358 and was found that the conjugation of CPP enhanced the intracellular uptake. These TAT conjugated to dextran coated IONPs, caused reactive oxygen species production when exposed to alternating magnetic field and resulted cell death. The treatment also caused the destabilization of the lysosomal membrane and eventually apoptosis of the cells [50]. Gold shell has been created at the surface of magnetic NPs, derivatized with dithiocarbamate. The C-terminus of the AMP (KWKLFKKIGAVLKVLC) was used for the immobilization of the peptide on gold surface (AMP-NPs).These NPs have been tested against E. coli and S. aureus. Results suggested that these NPs were internalized by phagocytes as well as by endothelial cells, and caused no inflammatory reaction at a concentration upto 200 μg/mL [51].
3. NANOMATERIALS FOR GENE DELIVERY
This section deals with different nanomaterials used for the delivery of genetic materials.
3.1. Metallic Nanoparticles
AuNPs functionalized with PEP peptide were studied for their transfection activity in the mesenchymal stem cells. Both the transfection activity for Vascular Endothelial Growth Factor (VEGF) and antibacterial activity were found to be greatly enhanced with the increase in the concentration of the peptide [52]. Unimer polyion complex-assembled (uPIC) AuNPs attached to cRGD peptide (cRGD-AuNPs-uPIC) were used for the delivery of siRNA. These peptides helped in effective targeted delivery of siRNA in HeLa cell lines [53]. CD4+ and CD25+regulatory T-cells (Tregs) play an important role in maintaining the immunogenic responses in the body. AuNPs conjugated with eGFP-siRNA penetrated inside Tregs and caused a reduction in eGFP expression and modulated the genetic expression [54]. Quercetin attached to AgNPs was tested for antimicrobial activity against various pathogenic microbes (AgNPs-Qe). These AgNPs-Qe were stabilized with siRNA. The siRNA/AgNPs-Qe were found effective in silencing the targeted gene and caused reduction in bacterial propagation. The intravenous injection of siRNA/ AgNPs-Qe to nude mice showed a decrease in bacterial content in blood [55]. One-step synthesis was employed for the preparation of AgNPs using chitosan-g-polyacrylamide. These NPs were stabilized using PEG and functionalized with RGDS peptide. The DNA transfection ability of NPs was found to be greatly enhanced in HeLa and A549 cells with minimal cellular toxicity [56]. The Se@MIL-101 and Ru@MIL-101NPs were formed by linking Se and Ru to Fe attached with siRNA. These NPs were tested against multi-drug resistant bacteria. In MCF-7/T,these NPs caused silencing of MDR genes, which led to enhanced apoptosis and instability of microtubules in cellular cytoskeleton [57].
3.2. Polymeric Nanoparticles
Cationic polymer polyethylenimine (PEI) and glycol chitosan have been reported to encapsulate siRNA. These formed complexes were found to be stable and inhibited Red Fluorescent Protein (RFP) gene expressions in cells and mice bearing tumors [58]. The synergistic effect for gene expression and cancer treatment has been assessed by treating the cells with nano-carriers of PEI and PCL. Both PEI and PCL were encapsulated with Dox and siRNA, which were surface modified with poly(ethylene glycol)-block-poly(glutamic acid) for targeted delivery to cancer cells. The cancer cells showed enhanced delivery of the BCL-2 siRNA showing RNA interference with increased Dox activity [59]. The surface of the dendrimer was modified with a peptide RGD and AuNPs were entrapped in it.The naïve, the modified dendrimers as well as the dendrimers encapsulating AuNPs were used as a carrier to transfer pDNA in human mesenchymal cells. This pDNA encoded human bone morphogenetic protein-2 (hBMP-2) gene, green fluorescent protein and luciferase. The PEG-RGD entrapped AuNPs dendrimer was found to be more efficient in transfecting hBMP-2 gene [60]. AgCHS (Chitin synthase gene), a double-stranded RNA was delivered using chitosan NPs in order to increase its retention stability as well as efficacy in the gut epithelial cells of larva. The larva of Anopheles gambiae when fed with chitosan/AgCHS NPs suppressed AgCHS1 and AgCHS2 genes, responsible for synthesis of chitin. The reduction in chitin level was found to be 38% and larvae were more susceptible to different pesticides compared to control [61]. The rat skeletal muscle was injected with positively charged lipids microbubbles with DNA attached to them. On exposing to ultrasound waves, the DNA released from bubbles and deposited in the skeletal muscle tissues. An enhanced gene expression has been found for echo contrast bubbles as compared to the naked DNA [62]. A polycationic NP composed of 2-(diethylamino)ethyl methacrylate were prepared by loading anionic siRNA in it. The in vitro efficacy was evaluated in embryonic kidney cell line HEK293T and the murine macrophage cell line RAW264.7. These cell lines showed an increase internalization of siRNA [63]. For transdermal delivery of DNA, NPs of chitosan and poly-g-glutamic acid were prepared. Compared to the control; the chitosan NPs with DNA showed enhanced gene expression in the mouse skin due to increased penetration [64]. The encapsulation of luciferase targeted siRNA was done in PLGA polymer. The in vitro efficacy was higher for silencing of the model gene, luciferase in MDA-kb2 cell lines [65].
3.3. Liposomes
Liposomes conjugated with bi-ligands have been exploited for the delivery of genes to cross the BBB. The β-gal plasmid was loaded into bi-ligand (transferrin-poly-L-arginine) liposomes and were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine iodide(DiR). The bi-ligand liposomes crossed the brain and showed enhanced expression of β-gal in the brain tissue of rat as compared to transferrin ligated liposome [66].The fibrosis can be decreased by knocking out the Myocardin-Related Transcription Factors (MRTF). A receptor-targeted liposome-peptide-siRNA NPs has been designed for silencing MRT and to treat conjunctival fibrosis. Two different liposomes LYR (non-PEGylated liposome-peptide Y-siRNA) and LER (non-PEGylated liposome-peptide ME27-siRNA) were found to be effective in causing approximately 76% silencing of the MRTF-B. This effect was found to be increased when compared with siRNA alone or the non-targeting peptides [67]. The liposomes loaded with VEGF siRNA and doclitaxel were surface modified with Angiopep-2 and neuropilin-1 receptor (tLyP-1) peptides. The effect of these liposomes were observed in human glioblastoma cells (U87 MG) and murine Brain Microvascular Endothelial Cells (BMVEC). These modified liposomes showed augmented penetration, gene silencing and antiproliferative activity [68].A Multi-Functional Nano Device (MEND) was synthesized for the in vivo delivery of siRNA to the Tumor Endothelial Cells (TEC). The MEND made up of a cationic lipid YSK05 was loaded with TEC siRNA which was further attached with cyclic RGD peptide. The cRGD aided in targeted delivery of the MEND towards TEC cells and caused gene silencing [69].The liposomal-siRNA, surface modified with PEG linked to hyaluronic acid (PEG-HA-NP)were reported for enhanced delivery of siRNA. The increase in siRNA delivery was due to fast degradation of hyaluronic acid in endosomes, causing an endosome escape and finally siRNA release. The p-glycoprotein expression on the surface of MCF-7 cells has been downregulated by the transfection of anti-P glycoprotein siRNA and showed better silencing [70]. The tumor model generated in mice was treated with pDNA encoding firefly luciferase. The tumors treated with lipid-pDNA along with ultra-sonication showed maximum levels of transfection as compared to conventional transfection method. Same results have been reported when the tumors were treated with pDNA IL-12 gene using lipids. There was suppression in the growth of the tumors [71].
3.4. Magnetic Nanoparticles
The MNPs have been well explored as transfecting agents. A technique termed as magnetofection has been introduced which works on the principle of combining the genetic material with MNPs. For the in vitro transfection, the MNPs along with the genetic material are added to the cell culture where the effect of magnetic field generated by the metals causes an increment in the transfection rate. In case of in vivo studies, the magnetic field generated causes both the targeted delivery as well as the speed of transfection. By altering the magnetic fields, the release of the genes at the targeted site has been achieved [72]. HeLa cell line induced with reporter gene luciferase was evaluated for the knockdown activity by the magnetofection of siRNA [73]. An antisense oligodeoxynucleotides have been delivered in HUVEC as well as in femoral arteries of mice. The transfection of oligodeoxynucleotides was enhanced by 84%. The effect of oligodeoxynucleotides and siRNA has been shown in the signaling pathway where NADPH oxidase activity was reduced by Sh-2 domain of phosphatase 1 in HUVEC cells [74]. For targeted delivery of DNA, heparin sulfate linker was used to attach Adeno-Associated Virus (AAV) encoding Green Fluorescent Protein (GFP) at the surface of MNPs [75]. The IONPs were coated with lipids in order to increase their stability by adding a solvent N-methyl-2-pyrrolidone (NMP) to stimulate adhesion. The cationic lipid coating on the surface of NPs was exploited to adsorb DNA and siRNA on its surface by electrostatic interaction. The in vitro effectiveness evaluated in HeLa cell lines and the transfection for DNA delivery was found to be maximum for 50-100 nm sized NPs whereas 40 nm sized NPs were appropriate for delivery of siRNA [76].The IONPs containing plasmid DNA were surface coated with PEI and further attached with bis(cysteinyl) histidine-rich TAT peptide. The synergistic effect of magnetofection and TAT mediated-delivery was enhanced by 4 times both in vitro as well under in vivo conditions [77].
CONCLUSION
The hunt to design advanced nanomaterials for targeted delivery of biologically important molecules has been gaining tremendous interest amongst researchers. The delivery of proteins, peptides and genes is very promising in performing various functions of the body. The impediment in the delivery of these molecules is their low stability, degradation by various enzymes in the biological milieu, less bioavailability, etc. Nanotechnology-based systems have been developed to address these problems and to increase the efficacy of these molecules. Further, specific delivery of the biomolecules to the infected sites via targeted approach can be also achieved using nanodelivery agents for the treatment of acute and chronic ailments. The encapsulation/conjugation has helped in maintaining the structural as well as functional identity of the molecules. The nano-encapsulated siRNA has been found to be more stable and their retention time has also been found to increase. But keeping in mind that “Technology-Yes, but Safety-Must”, prior to implementing this new class of delivery agents, we also need to evaluate the new kinds of toxicity concerns associated with these delivery agents.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to the Director, CSIR-IHBT for his constant support and encouragement. AA acknowledges financial assistance in the form of project grant MLP-0201 from CSIR and GAP-0214 (EMR/2016/003027) from DST, Government of India. AG is thankful to the Academy of Scientific and Innovative Research (AcSIR). The CSIR-IHBT communication number of this manuscript is 4250.








